Sep 06, 2019
Metabolically phenotyped surgical patients represent a novel and complementary source of pancreatic tissue for diabetes research
Contemporary research of beta cell biology in context of type-2 diabetes is based mainly on the long-standing technique for isolation of the pancreatic islets of Langerhans. This procedure, which involves the use of enzymes for the separation of the pancreatic endocrine cells from the surrounding exocrine tissue, may introduce unwanted effects on the former. Scientists of the Paul Langerhans Institute Dresden developed a new approach to isolate islets from snap frozen surgical specimens donated by metabolically phenotyped pancreatectomized patients. A new review article published in “Molecular Metabolism” explains how this platform may represent a paradigmatic shift for the study of human islets and diabetes research.
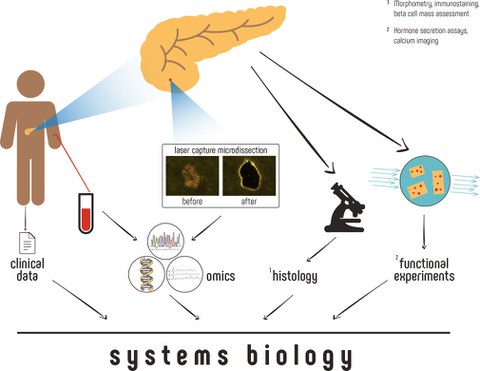
Type-2 diabetes mellitus affects millions of people worldwide and presents a growing issue and burden to the patients’ longevity and quality of life, as well as an increasing challenge to the healthcare systems. Defining feature of this disease is the failure of beta cells, which has been studied extensively over the past decades mainly using enzymatically isolated primary islet cells as a model system. However, the advent of high throughput “-omics” approaches has dramatically improved our ability to measure subtle changes in biomolecules. Thus, it has become possible to apply these technologies to surgical specimens as experimental models to study beta cells in situ, and thereby in a context that more faithfully represent their state in vivo.
“In our review, we discuss and present an approach for the collection of islet tissue of highest purity from limited amount of material that can be used for practically all downstream “-omics” applications. Our approach relies on the use of Laser Capture Microdissection (LCM), a technique that utilizes a laser beam to physically cut out the desired endocrine cells from the surrounding tissue” explains Marko Barovic, PhD student in the Solimena lab of the Paul Langerhans Institute Dresden and first author of the review. Using the LCM technique, islets can be isolated from only a few hundred micrograms of pancreatic tissue procured during routine processing of surgical specimens of patients undergoing pancreatic surgery. This approach also has the particular advantage of being applicable to virtually any academic hospital or research center performing pancreatectomies, thereby massively increasing the number of samples that can be collected nationwide. Snap freezing of the tissue directly after explantation prevents further changes of the biological material, thereby allowing subsequently downstream measurement of cellular parameters, such as mRNA or protein levels in a state that is almost identical to that inside the human body. “A further and highly valuable advantage of our approach is the access to all relevant clinical information and the possibility to perform glucose challenge tests immediately prior to surgery for accurate metabolic phenotyping of the donors. For the first time, we have therefore the possibility to combine clinical data with deep “-omics” profiling, and thus to track the observed changes in relation to the duration and progress of the disease”, summarizes Barovic.
Methods presented in the review are being actively used in collaborative efforts within the IMI Consortium RHAPSODY, striving to provide a broad insight into the molecular characteristics of diabetes on European level.
Original Publication:
Barovic M, Distler M, Schöniger E, Radisch N, Aust D, Weitz J, Ibberson M, Schulte AD, Solimena M. Metabolically phenotyped pancreatectomized patients as living donors for the study of islets in health and diabetes. Mol Metab. 2019 Sep;27S:S1-S6. doi: 10.1016/j.molmet.2019.06.006. Review.
https://www.sciencedirect.com/science/article/pii/S2212877819305678