May 13, 2025
New insights into how insulin mRNA is stored in beta cells during resting blood-glucose levels.
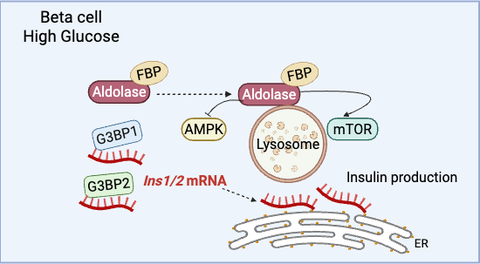
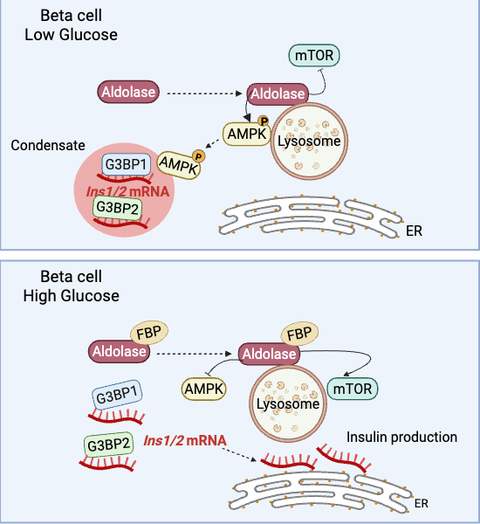
Type 2 Diabetes (T2D) is a condition that causes insufficient production of insulin, the glucose regulating hormone secreted by the pancreas. During an influx of glucose in the bloodstream, beta cells of the pancreas translate pre-existing insulin transcript variants (Ins1/2 mRNA) to produce the hormone. A current study by a research team led by scientists of the Paul Langerhans Institute Dresden (PLID) of the German Center for Diabetes Research investigated the mechanisms by which murine beta cells store Ins1/2 mRNA during resting blood glucose levels, which resemble a state of fasting. The researchers found that the RNA binding protein G3BP1, which is downregulated in pancreatic islets of T2D patients compared to normoglycemic donors, compartmentalizes insulin mRNA into cytosolic condensates under fasting conditions. Consequently, when cells are exposed to stimulating glucose concentrations (i.e., blood glucose levels after a meal), these intracellular condensates dissolve, thus enabling immediate insulin mRNA translation into the insulin hormone. This research, recently published in EMBO Journal, provides novel insights about the storage of insulin mRNA, which has been enigmatic in the field for many years, and raises the possibility that this process may be impaired in patients with T2D.
Pancreatic beta cells are the body’s producer of insulin, the peptide hormone responsible for facilitating glucose uptake into cells. Impaired beta cell function and insulin resistance are the main factors causing T2D. Patients suffering from T2D therefore have trouble with the uptake of glucose into their cells, as they cannot produce enough insulin to regulate high blood sugar levels. Previous research has found that the newly synthesized insulin molecules in beta cells as a result of a blood sugar spike does not depend on increasing insulin gene expression, but rather an increment in the translation of pre-existing Ins1/2 mRNA transcripts. This indicates that insulin mRNA is stored somewhere in the cytosol of beta cells when it is not needed, for example, during fasting. How and where beta cells store Ins1/2 mRNA, however, remains a mystery.
“In a previous study, transcriptomic analyses of laser captured microdissected pancreatic islets of different donors showed that G3BP1 is downregulated with the highest score in patients with T2D”, explains Esteban Quezada, a post-doctoral researcher in the lab of Michele Solimena and first author of the study. “For this reason, we decided to take a closer look at how G3BP1, and its paralog G3BP2, may be involved in Ins1/2 mRNA storage.”
To address this, a research group comprising scientists from various institutions and under the leadership of Michele Solimena from the PLID investigated the possible relationship of insulin mRNA storage and the stress granule marker G3BP1 during fasting. Stress granules are cytosolic compartments composed of RNA-binding proteins and several mRNAs that form during different stress conditions. While resting glucose levels are not a stress condition but rather a physiological state, thus similar machinery could be used by beta cells to confine Ins1/2 mRNA in response to resting glucose.
This new study mainly performed in MIN6-K8 cells found that Ins1/2 mRNA was confined into G3BP1/2-positive condensates during resting conditions. Interestingly, when the cells were exposed to higher glucose levels the condensates disassembled and the components were redistributed throughout the cytosol. A closer look also made evident that G3BP1, but not G3BP2, contributes to the stability of Ins1/2 and to proinsulin production. Importantly, the presence of G3BP1/2-positive condensates confining insulin mRNA was validated in mouse primary beta cells and in human pancreatic tissue sections. These findings point towards the idea that decreased G3BP1 levels may play a significant role in T2D pathology and the mechanism in which insulin production is being hindered in these patients. Prof. Solimena concludes that, “further understanding of this mechanism could prove to be a useful tool in the complex treatment of T2D,” and plans to continue the investigation of this topic.
Original Publication
Esteban Quezada, Klaus-Peter Knoch, Jovana Vasiljevic, Annika Seiler, Akshaye Pal, Abishek Gunasekaran, Carla Münster, Daniela Friedland, Eyke Schöniger, Anke Sönmez, Pascal Roch, Carolin Wegbrod, Katharina Ganß, Nicole Kipke, Simon Alberti, Rita Nano, Lorenzo Piemonti, Daniela Aust, Jürgen Weitz, Marius Distler, and Michele Solimena. Aldolase-regulated G3BP1/2+ condensates control insulin mRNA storage in beta cells. https://doi.org/10.1038/s44318-025-00448-7