31.01.2024
Neue Expansionsmethode verbessert Versorgung mit Vorläuferzellen der Bauchspeicheldrüse
In einer für die regenerative Medizin zukunftsweisenden Entwicklung haben Forscher des Paul-Langerhans-Instituts Dresden die Mechanismen identifiziert, die die Expansion und Differenzierung von Vorläuferzellen der Bauchspeicheldrüse (Pankreasprogenitorzellen, PP) regulieren. Dies ermöglichte die unbegrenzte Vermehrung von PP-Zellen, die aus humanen pluripotenten Stammzellen (hPS-Zellen) stammen. Die Ergebnisse dieser Arbeit wurden jetzt in "Elife" veröffentlicht.
Diabetes mellitus, von dem fast 10 % der Weltbevölkerung betroffen sind, tritt in zwei Hauptformen auf, die durch die immunologische Zerstörung (Typ 1) oder die Fehlfunktion (Typ 2) der insulinproduzierenden Betazellen in den Langerhans-Inseln der Bauchspeicheldrüse verursacht werden. In schweren Fällen dieser Erkrankung ist oft eine Transplantation der gesamten Bauchspeicheldrüse oder der Inselzellen erforderlich, was jedoch aufgrund der Knappheit an Spendern und der Notwendigkeit einer lebenslangen Immunsuppression eine erhebliche Herausforderung darstellt. Aus diesem Grund wird derzeit gezielt daran gearbeitet, das Potenzial von hPS-Zellen zur Gewinnung von Inselzellen der Bauchspeicheldrüse, den so genannten SC-Inseln, nutzbar zu machen, die eine unbegrenzte Quelle von insulinproduzierenden Betazellen für die Transplantation darstellen könnten. Nun hat die Gruppe von Prof. Anthony Gavalas am Paul-Langerhans-Institut Dresden bemerkenswerte Fortschritte bei der Expansion von aus hPS-Zellen abgeleiteten PP-Zellen gemacht, ein entscheidender Schritt für die klinische Anwendung von SC-Inseln in der Diabetes-Zelltherapie.
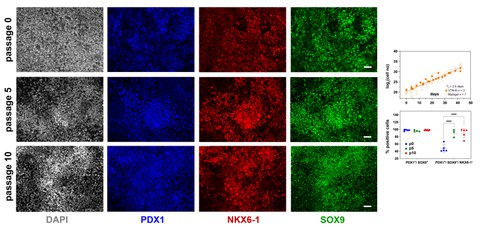
Nach ihren kürzlich veröffentlichten Ergebnissen bestand die größte Herausforderung ihrer Arbeit darin, die Selbsterneuerung von PP-Zellen aufrechtzuerhalten und gleichzeitig ihre Differenzierung zu hemmen. Die Gruppe ermittelte spezifische Bedingungen, die eine 2000-fache Expansion von PP-Zellen über 10 Passagen und 40-45 Tage ermöglichten und ein reproduzierbares und GMP-konformes Verfahren darstellten. Das chemisch optimierte Expansionsmedium entkoppelte die PP-Proliferation von der Differenzierung, indem es spezifische mitogene Signalwege stimulierte, während es die Retinsäure-Signalgebung und ausgewählte Zweige der TGFβ- und Wnt-Signalgebung unterdrückte. Das spezifische Medium förderte nicht nur die robuste Expansion von PP-Zellen, sondern auch deren effiziente Anreicherung, wie die Ergebnisse der Durchflusszytometrie für PDX1+/SOX9+/NKX6-1+ PP-Zellen zeigen. "Dieses Expansionsverfahren wird die Erzeugung von SC-Inseln für die weitere Entwicklung, die Diabetesforschung, die personalisierte Medizin und Zelltherapien erleichtern", kommentiert Luka Jarc, der Erstautor der Studie, und fährt fort: "Die expandierten PP-Zellen zeigten eine hohe Effizienz und Reproduzierbarkeit bei der Differenzierung in SC-Inseln, die funktionelle Betazellen enthalten, wie wir durch Tests zur glukosestimulierten Insulinsekretion nachgewiesen haben."
Darüber hinaus macht die Anwendbarkeit der Methode auf verschiedene hPS-Zelllinien und ihre Kompatibilität mit chemisch definierten Kulturmedien sie zu einer vielseitigen und skalierbaren Methode für die Produktion von PP-Zellen in großem Maßstab. Die Forscher glauben, dass dieser Durchbruch die Variabilität im Differenzierungsprozess verringern und die Auswahl optimal differenzierter PP-Zellpopulationen erleichtern wird.
Die Forscher gehen davon aus, dass die Technologie eine entscheidende Rolle bei der weiteren Entwicklung der regenerativen Medizin unter GMP-Bedingungen (Good Manufacturing Practice) spielen wird. Die Verfügbarkeit einer hochreinen, GMP-gerechten PP-Zellpopulation, die von hPS-Zellen abgeleitet ist, ist vielversprechend für zukünftige klinische Anwendungen und könnte einen wesentlichen Beitrag zur Bewältigung der Herausforderungen der Diabetesbehandlung leisten. "Das von uns entwickelte chemisch definierte Expansionsverfahren ist ein wichtiger Schritt auf dem Weg zur Erzeugung einer großen Zahl menschlicher endokriner Zellen der Bauchspeicheldrüse, die für die biomedizinische Forschung und die regenerative Medizin von großem Interesse sind", schlussfolgert Prof. Gavalas und verweist auf die weitreichenden Auswirkungen ihrer bedeutenden Arbeit.
Dieser innovative Ansatz ist ein wichtiger Meilenstein auf der Suche nach skalierbaren Lösungen für Diabetes-Zelltherapien und gibt Hoffnung auf bessere Behandlungsmöglichkeiten und Fortschritte in der regenerativen Medizin. Die Gruppe hat die Methode zum Patent angemeldet und mit Stem Cell Technologies eine Vertraulichkeitsvereinbarung zur kommerziellen Entwicklung dieses Verfahrens getroffen.
Originalpublikation
Jarc L, Bandral M, Zanfrini E, Lesche M, Kufrin V, Sendra R, Pezzolla D, Giannios I, Khattak S, Neumann K, Ludwig B, Gavalas A. Regulation of multiple signaling pathways promotes the consistent expansion of human pancreatic progenitors in defined conditions. Elife. 2024 Jan 5;12:RP89962. doi: 10.7554/eLife.89962.