Jun 27, 2024
New study unveils that a hierarchy of beta cells guides glucose responses in the islet, supported by Vitamin B6
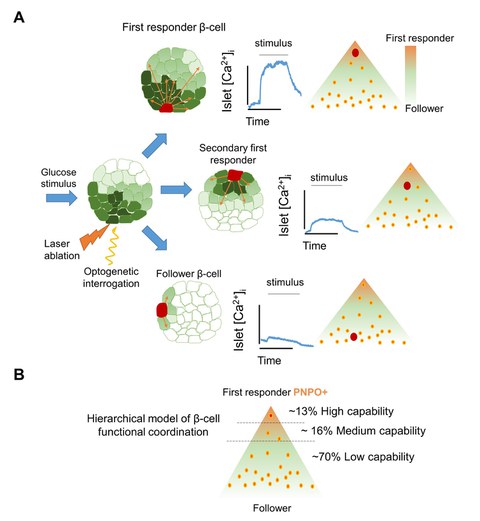
A) The functional characterization of islet coordination using optogenetics shows a hierarchical model for beta cell coordination. Coordination includes first responder cells at the top and follower cells at the bottom. B) First responder beta cells have a higher expression of the vitamin B6 production enzyme pnpo. They can trigger a Ca2+ response in many cells, while other beta cells have a medium to low ability to coactivate.
The coordination of cellular activity through calcium ions enables beta cells to secrete precise quantities of insulin. Now, an international research team led by scientists from the Paul Langerhans Institute Dresden (PLID) of the German Center for Diabetes Research implement optogenetic systems to explore in the zebrafish model how the Ca2+ response is orchestrated in space and time and which role individual beta cells play in the reaction to a glucose stimulus. This study, recently published in the journal Science Advances, has revealed crucial insights into the functional hierarchy of pancreatic beta cells in response to glucose, emphasizing the role of Vitamin B6 in this process. Moreover, it provides a novel understanding of how beta cells coordinate insulin secretion, a key factor in managing blood glucose levels.
Pancreatic beta cells regulate glucose homeostasis by secreting insulin in response to nutrient intake. Thereby, the insulin release occurs in two phases: an initial rapid increase in intracellular Ca2+ levels and insulin release, followed by a sustained, pulsatile insulin secretion. Individuals with prediabetes or type 2 diabetes (T2D) show an impaired first-phase secretion and reduced second-phase secretion, with the pulsatility of secretion also lost in T2D. Hence, understanding these mechanisms is crucial for developing effective novel diabetes therapies.
For this study, Prof. Nikolay Ninov’s research group at the PLID and the Center for Regenerative Therapies Dresden (CRTD) at TU Dresden teamed up with colleagues from the Université Libre de Bruxelles and the Imperial College London. The scientists employed optogenetic systems to probe the role of individual beta cells in zebrafish. This innovative approach allowed the team to manipulate beta cell activity using lasers and observe the resulting effects on Ca2+ dynamics across the islet. By targeting specific beta cells for activation or inactivation, they uncovered a hierarchical structure among beta cells, identifying a subset of "first-responder" cells. The team found that when the first responder cells were inhibited, the Ca2+ response of the islet was diminished. Conversely, when the first responders were selectively activated, the Ca2+ response of the islet was enhanced. Interestingly, only about 10% of the beta-cells in an islet are first-responders, suggesting that this small population of cells serves as a control center for regulating the activity of the rest of the islet’s beta-cells. The name first-responder denotes the ability of these cells to respond quicker to glucose than the rest of the beta-cells i.e., the follower cells. Notably, the closer a beta-cell is to the first-responder, the faster it tends to respond to glucose, thereby establishing a cellular hierarchy.
Furthermore, the work also highlighted for the first time the importance of Vitamin B6 in this process. With the help of the new Ca2+ reporter CaMPARI, a photoconvertible protein that converts from green to red fluorescence in the presence of high Ca2+ concentration, the researchers found that first-responder beta cells have distinct metabolic and molecular characteristics, including the expression of the enzyme pyridoxamine 5'-phosphate oxidase (pnpo), which is involved in producing the metabolically active form of Vitamin B6. Additional experiments confirmed that Vitamin B6 is essential for the coordinated Ca2+ response to glucose in both zebrafish and mouse islets. These findings suggest that Vitamin B6 plays a critical role in enabling the Ca2+ response to glucose and that possibly the first responder cells produce and provide Vitamin B6 to the rest of the beta-cells to regulate their activity. This could have implications for understanding the metabolic pathways that support insulin secretion and potentially for developing new treatments for diabetes.
The study's innovative use of optogenetic tools and intravital microscopy in zebrafish provided a unique advantage, allowing researchers to observe beta cell activity in a living organism while preserving normal physiological conditions. This approach revealed that first-responder cells exhibit a higher glucose sensitivity and a distinct gene expression profile compared to other beta cells. "Our research underscores the importance of beta cell heterogeneity and the division of labor within the islet," said Nikolay Ninov. "Understanding the specific roles of different beta cell subpopulations could lead to more targeted and effective therapies for diabetes in the future. And I am convinced, that the insights gained from this study open new avenues for exploring the mechanisms of beta cell coordination and their role in glucose homeostasis.”
Original publication
Delgadillo-Silva, L. F., Tasöz, E., Singh, S. P., Chawla, P., Georgiadou, E., Gompf, A., Rutter, G. A., & Ninov, N. (2024). Optogenetic β-cell interrogation in vivo reveals a functional hierarchy directing the Ca2+ response to glucose supported by vitamin B6. Sci. Adv. 10, eado4513 (2024)
