Schubert group
In vitro disease modeling using microphysiological systems
In collaboration with Fraunhofer IWS (Dr. F. Sonntag)
https://www.iws.fraunhofer.de/de/technologiefelder/additive-fertigung-und-oberflaechentechnik/mikro-biosystemtechnik.html)
Contact: Dr. Mario Schubert, Prof. Dr. Kaomei Guan
Staff: Dr. Mario Schubert, M.Sc. Oliver Gamm, M.Sc. Yuliya Dzekhtsiarova
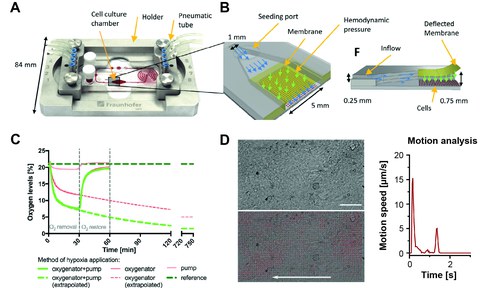
In this collaborative project with the Fraunhofer IWS, we established a cell culture chip system, or microphysiological system (MPS), for the cultivation of induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs). The MPS represent a closed cultivation system with circulating culture medium integrated micropump and oxygenator. A control platform allows regulation of the flow rate of the medium in the chip and of oxygen levels in the culture chamber of the iPSC-CMs. During cultivation, the iPSC-CMs can be observed using transmitted light and fluorescence microscopy, which enables the use of diverse biological readouts such as reactive oxygen species, mitochondrial morphologie or cell viability. The contractile function of the cells can be characterized using high-speed imaging. Using the MPS, we can recapitulate complex biological processes and simulate pathophysiological scenarios in vitro. Our current focus aims on the simulation of an acute myocardial infarction and the associated ischemia-reperfusion damage.
Figure 1: Microphysiological system (MPS) for cultivation of iPSC-CMs and disease modeling . A, B, structure of the MPS in support (A) and detailed representation of the cell culture chamber (B) with semi-permeable membrane, medium flow and hemodynamic stimulation. C, Regulation of oxygen content in the culture medium to induce hypoxia.
D, Video-based analysis of cardiomyocyte function in the MPS (Kolanowski et al., 2020).
Development of a human Multi-Organ-on-Chip System to investigate the function of monocytes and macrophages after ischemia-reperfusion injury
The project is funded by the German Federal Institute for Risk Assessment Grant Agreement Number 60-0102-01#00067 - P639.
Contact: Dr. Mario Schubert, Prof. Dr. Kaomei Guan
Staff: M.Sc. Shakthi Arun, M.Sc. Oliver Gamm, B.Sc. Pranotee Gawade, Paul Beck, M.Sc. Yuliya Dzekhtsiarova
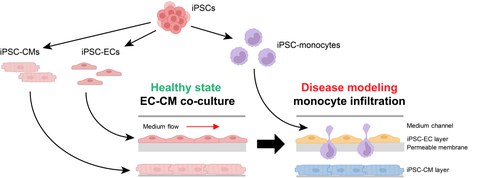
Acute myocardial infarction (MI) and the resulting development of heart failure (HF) is one of the leading causes of death or impairment of quality of life. The response of the immune system, particularly monocytes and macrophages, is of critical importance for the size of the infarct area, and represents a starting point for the development of new therapies. Animal MI-models can only partially recapitulate the immune reaction and are associated with a substantial worsening in the general condition and death of the animals. With the development of a multi-organ-on-a-chip system (MOoC) based on human cell models, we aim to close this gap in translational research and to replace animal models. The MOoC system enables the cultivation of a complex heart tissue model; consisting of cardiomyocytes, fibroblasts and resident macrophages; the integration of an endothelial barrier as well as the circulation of monocytes and macrophages. The ischemiareperfusion (I/R) injury, which occurs during MI, will be simulated with the MOoC to examine the infiltration of monocytes and macrophages into the damaged heart tissue. The aim is to analyze the role of different monocyte and macrophage subtypes in the inflammatory response, tissue repair and development of HF. the application of human induced pluripotent stem cells to produce the cell types enables us to establish a MOoC completely based on human cell models. The effective in vitro simulation of the clinically relevant scenario of monocyte and macrophage infiltration after I/R injury with this human MOoC system offers great potential for replacing animal models in various research areas, beyond the question of heart damage after MI.
Figure 2: Induced pluripotent stem cell (iPSC)-based human multi-organ-on-chip system (MOoC) to investigate the infiltration of monocytes in damaged heart tissue. Cardiomyocytes (iPSC-CM), endothelial cells (iPSC-EC) and monocytes are differentiated from iPSCs. ECs form a functional membrane barrier and CMs a heart tissue model inside the MOoC. Incubation under hypoxia leads to damage of EC and CMs, and iPSC-derived monocytes are applied into the circulating medium inside the MOoC, to study their infiltration into the injured heart tissue.
Interaction of cardiomyocytes and cardiac fibroblasts in development and pathophysiology
Formerly funded by MeDDrive Grant der TU Dresden (to Dr. M. Schubert).
Contact: Dr. Mario Schubert, Prof. Dr. Kaomei Guan
Staff: Wilhelm Wenke, Paul Josef Conrad Beck, B.Sc. Isha Bansal
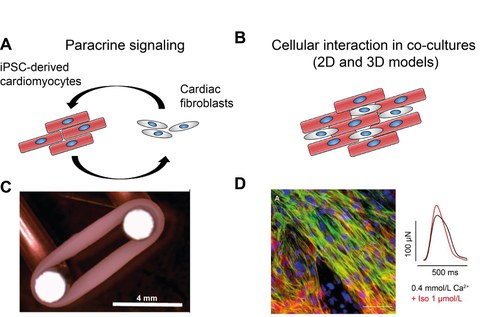
Cardiac fibroblasts play a key role for physiological and pathophysiological processes in the heart, such as heart development or the development of cardiac fibrosis. However, our understanding of the underlying molecular mechanisms involved in the cell-cell interactions between different cardiac cell types is very limited. Better knowledge these processes will, very likely, pave the way for the development of new treatment strategies and identification of novel pharmacological targets. For this reason we are investigating the interaction of human iPSC-CMs and cardiac fibroblasts. In our studies, we appy 2D cell culture models as well as 3D tissue systems, including engineered heat tissues (EHTs) or cardiac organoids, consisting of several cardiac cell types (cardiomyocytes, fibroblasts, endothelial cells). These co-culture models enable us to investigate the development of fibrosis and its impact on cardiac tissue function in vitro.
Figure 3: Investigation of cardiomyocyte-fibroblast interactions. A, B, The bi-directional crosstalk of both cell types occurs through paracrine signaling pathways (A), based on soluble factors or extracellular matrix, or direct cell-cell contacts (B). C, D, Engineered heart tissue (EHT) mounted on a stretcher for maturation of the tissue (C). EHTs are formed from iPSC-CMs and human ventricular fibroblasts and provide direct cell-cell interactions and enable the analysis of cellular structures as well as measurement of the contraction force under pharmacological stimulation (D).
Maturation of iPS-cardiomyocytes in 2D and 3D models for drug testing and disease modeling
Formerly funded by MeDDrive Start der TU Dresden (to Dr. M. Schubert).
Contact: Dr. Mario Schubert, Prof. Dr. Kaomei Guan
Staff: Paul Josef Conrad Beck, M.Sc. Oliver Gamm
A major limitation in drug development represents the limited of translatability of pre-clinical therapetic concepts, identified in mouse models, to their clinical application in patients. The establishment of human model systems is one of the key challenges for the reduction as well as the replacement animal experiments and to improve the success rate for clinical translation. The production of human cardiomyocytes by cardiac differentiation of induced pluripotent stem cells (iPSC-CMs) provides an unlimited source of human myocardial cells as model systems for biomedical research and drug screening. However, a crucial limitation represents the embryonic, immature phenotype of the iPSC-CMs, which limits their predictive value with respect to risk evaluation of cardiotoxic or arrhythmogenic agents. Thus, the focus of this project is to improve maturation state of iPSC-CMs in terms of their functionality, metabolism and cell structure with the aim to establish a cell model which recapitulates the properties of human adult heart muscle cells. For this purpose, we use diverese 2D- and 3D-models combined with application of various stimuli and characterized the iPSC-CMs using a vartiety of different methods to examine the function (contractile, electrophysiological), metabolism (Seahorse, Oroboros, gene expression) and structure (immunostaining, qPCR, western blot).
Methods:
- Differentiation of human induced pluripotent stem cells into cardiomyocytes
- Genome editing using CRISPR-Cas9
- Real-Time PCR, Western Blot, immuncytochemistry
- Fluorescence microscopy, live-cell monitoring of calcium transients, calcium sparks, action potentials
- Flow cytometry
- Seahorse technology, Oroboros
- Video based contraction analysis
- Cultivation and characterization of Engineered Heart Tissues
- Microphysiological cell culture system (collaboration Fraunhofer IWS)
People
Dr. Mario Schubert – Junior group leader
Phone: +49 351 458 6421
Email:
Paul Beck – MD student
Philipp Hartmann – MD student
Oliver Gamm – Phd student
Shakhti Arun - Phd student
Isha Bansal - Master student
Pranotee Gawade - Master student
Niclas Speri - MD student
Wilhelm Wenke – MD student
Sinah Hansen – MD student
Alumni:
Robert-Patrick Steiner – MD Thesis
Marcel Hasse – MD Thesis