Kämmerer Group
Research Focus
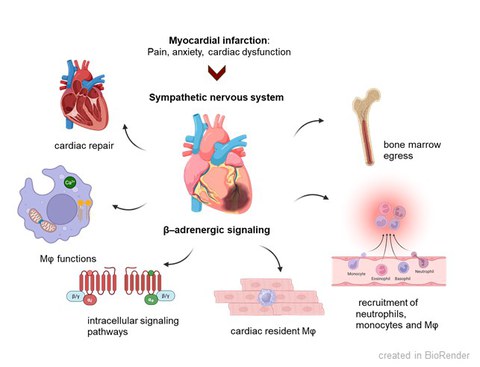
Cardiovascular diseases are among the most common diseases worldwide. As a result of other diseases such as high blood pressure or diabetes mellitus, many patients suffer a heart attack and develop cardiac muscle weakness with a reduced pumping function of the heart. The acute ischemia during myocardial infarction (MI) initiates complex inflammatory and neurohormonal responses. The stimulation of the innate immune system is essential for sufficient healing, while a chronic activation becomes detrimental resulting in the expansion of infarct size and numerous structural and electrophysiological changes in the heart muscle (remodeling). After MI, the activation of the sympathetic nervous system (SNS) is provoked by pain, anxiety and cardiac dysfunction. In the early inflammatory phase after MI, the activated SNS induces leukocyte recruitment to the infarcted heart via β-adrenoceptors (β-AR). However, the impact of activated SNS on the cardiac innate immune response and cardiac repair after initial recruitment remains unclear.
Due to adverse left ventricular remodelling, patients develop a chronic heart failure and very often die from fatal arrhythmias and sudden cardiac death. In order to permanently improve heart function, various neurohormonal systems (sympathetic system, renin-angiotensin-aldosterone system) are activated in the body. However, this chronic activation promotes the remodeling in the heart and the progression of heart failure. Drugs that inhibit the activation of these neurohormonal systems are used to treat HI (e.g. beta blockers to inhibit the sympathetic β-adrenergic signaling pathway and RAAS inhibitors). New therapeutic strategies aim at the additional activation of protective mechanisms, e.g. the activation of the natriuretic peptide (NP) system. Despite these therapeutic advances, heart failure and fatal ventricular arrhythmias remain a leading cause of death worldwide. New innovative concepts are desirable to improve therapy.
Approach
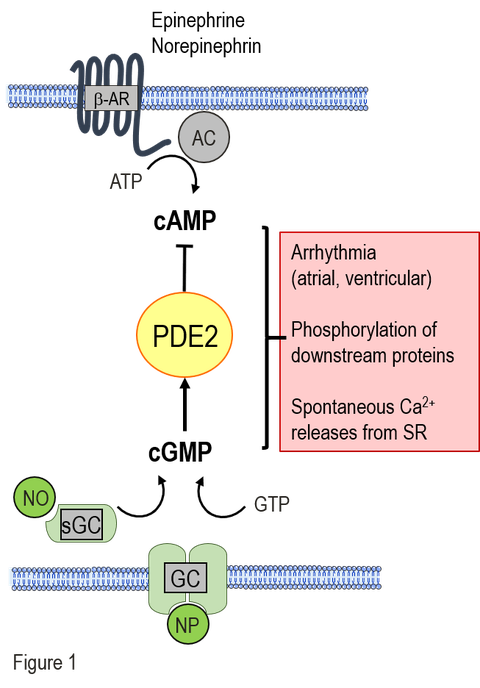
New antiarrhythmic strategies: A major subcellular reason is the chronic stimulation of the β-adrenergic cAMP-system as consequence of the reduced cardiac function in HF and the limited energy supply in diabetes. The permanent hyperactivity of the cardiac cAMP signaling cascade leads to proarrhythmic remodeling of the calcium homeostasis in cardiomyocytes. In contrast, the activity of the protective cGMP signaling pathway is low or diminished in chonic HF and diabetic cardiomyopathy. The phosphodiesterase 2 (PDE2) is a signaling node, connecting cAMP and cGMP signaling pathways. Among PDEs, it has a unique property to be stimulated by cGMP leading to a remarkable cAMP hydrolysis. Thus, PDE2 mediates a negative crosstalk between cGMP/cAMP pathways (see Fig. 1). Our aim is to decipher the function of PDE2 within the cardiac cGMP/cAMP crosstalk and to evaluate PDE2 as a potential target for antiarrhythmic therapy in chronic HF and diabetic cardiomyopathy.
To unravel the impact of PDE2 on cardiac function in cardiovascular and metabolic diseases, we generated mouse models with either cardiac specific PDE2 overexpression (PDE2 transgenic mice) or cardiac specific PDE2 deletion (PDE2 knockout mice). To study PDE2 functions under physiological and pathophysiological conditions, we combine different in vivo and in vitro techniques like echocardiography, ECG-telemetry, histology, patch-clamp technique, Calcium imaging, qPCR and western blot.
New anti-inflammatory strategies: After acute MI, the activated SNS modulates the innate immune response via β-AR. In the early inflammatory phase after MI, β-AR signalling regulates leukocyte recruitment to the infarcted heart. The role of β-AR signalling in specific cardiac immune cell populations within the reparative phase after MI remains unknown. Published data are controversial indicating anti-inflammatory effects of β-AR signalling in macrophages likely mediated by cAMP and limited by phosphodiesterases, while others suggest pro-inflammatory signalling enhancing cytokine levels. We aim to elucidate how β-adrenergic signalling regulates inflammatory responses, immune cell functions and cardiac repair following MI in both murine models and human patients.
Ongoing Projects
In our current projects on anti-arrhythmic strategies, we investigate the effects of PDE2 stimulation by natriuretic peptides on intracellular arrhythmia-triggering mechanisms and the development of arrhythmias. Natriuretic peptides mediate their cellular effects via membrane-associated guanylate cyclases (GC), which generate intracellular cGMP and can thus increase PDE2 activity. In cardiomyocytes from healthy mice, we have demonstrated that PDE2 stimulation by C-type natriuretic peptide (CNP) leads to a reduction in pro-arrhythmic spontaneous calcium releases and action potentials. Furthermore, CNP was able to reduce the development of arrhythmias in vivo after arrhythmia provocation. In the current projects, we are evaluating the potential of CNP-mediated PDE2 stimulation as a therapeutic principle to reduce arrhythmias in the following diseases:
-
atrial fibrillation
-
after acute myocardial infarction
-
during heart failure
-
in diabetic cardiomyopathy
In our current projects on anti-inflammatory strategies, we will identify immune cell populations with prominent β-adrenergic signalling after MI. We will decipher potential anti-inflammatory effects of β-AR in resident macrophages, and if this is mediated by cAMP/PKA signalling. We will further investigate pro-inflammatory β-adrenergic actions in recruited macrophages. Importantly, we will study sex-dependent differences of β-AR signalling in macrophages. Phosphodiesterases can also modulate cAMP levels in macrophages. Here, we will dissect the role of PDE2 in resident and recruited macrophages after MI to understand if it is either limiting proinflammatory or reducing anti-inflammatory β-AR signals.
Future Direction
Natriuretic peptides (NP) are secreted by various cell types under cardiac stress. NP mediate cardioprotective effects such as natriuresis, vasodilation, as well as antihypertrophic and antifibrotic effects. In addition, it was shown that ANP, BNP and CNP mediate anti-inflammatory effects. Thus, NPs inhibit monocyte and macrophage cytokine secretion, regulate monocyte chemotaxis, and reduce macrophage invasion into the myocardium. The underlying intracellular mechanisms are poorly understood. In future projects, we will investigate the influence of NP on different immune cells, such as monocytes and macrophages. We will evaluate the effects of NP-induced stimulation on cardiac inflammation, infarct size, and the occurrence of arrhythmias after acute myocardial infarction.