Klapproth Group
We are a young, interdisciplinary and diverse research team at the Institute of Pharmacology and Toxicology, Faculty of Medicine, TU Dresden. Our central research interests lie in deciphering disease-driving immune mechanisms after myocardial infarction and in developing sustainable biopharmaceutical production platforms for innovative therapeutics.
Immunopharmacological Research After Myocardial Infarction
After myocardial infarction, early immune processes not only determine the extent of inflammation but also shape the structural and functional future of the heart. Our research addresses this critical window by investigating how protease-controlled programs in neutrophils actively drive cardiac remodeling after infarction and how these processes can be therapeutically targeted with precision.
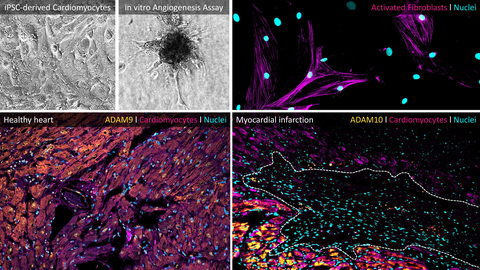
At the core of our work is the functional heterogeneity of neutrophils in the infarcted heart. Contrary to the classical view of neutrophils as short-lived, purely pro-inflammatory effector cells, our data demonstrate that neutrophils differentiate into distinct subpopulations. These subsets are less defined by canonical inflammatory mechanisms and instead characterized by their interaction with the extracellular matrix, intensive cell–cell communication, and tissue-modifying or tissue-destructive programs. These previously underappreciated neutrophil subtypes represent key drivers of post-infarction structural remodeling.
A particular focus of our research lies on ADAM metalloproteases - especially ADAM9 and ADAM10 - as well as the CX3CL1/CX3CR1 axis as molecular control points of immune cell recruitment, activation, and function. We do not consider proteases as passive biomarkers, but as active regulators of neutrophil identity and behavior within the tissue. Cell-type-specific protease targeting thus offers novel opportunities to precisely modulate inflammatory and tissue-remodeling processes in the heart without globally suppressing essential immune responses.
Methodologically, we combine genetic mouse models (including conditional knockouts), iPSC-derived human cell systems, CRISPR/Cas-based genome editing, multi-omics approaches (RNA-seq, proteomics, secretome analyses), high-dimensional flow cytometry, advanced imaging techniques, and functional cardiovascular in vivo models. This integrative strategy enables us to systematically dissect protease-driven immune mechanisms across molecular, cellular, and functional levels and to translate these insights into concrete therapeutic concepts.
GreenPharming – Sustainable Production of Therapeutic Proteins
In parallel, our group leads major parts of the institute-wide GreenPharming initiative. The goal is to establish plant-based, animal-free production systems for biopharmaceuticals such as antibodies, peptides, and cytokines. In the long term, these systems are intended to operate under GMP conditions within a circular bioeconomy in the Lusatia region.
We use plants as expression platforms, for example via transient gene transfer in Nicotiana benthamiana, and develop modular vector systems that enable rapid, flexible, and scalable protein production. Purified proteins are comprehensively characterized with respect to structure, glycosylation, and biological function, including mass spectrometry, HPLC, and functional cell-based assays. A particular focus lies on innovative cytokines for the expansion of CAR-T and CAR-NK cells for applications in personalized cell therapy.
The project combines biotechnological innovation with sustainability: plant biomass is reintegrated into circular value chains, renewable energy sources are utilized, and regional value creation in Lusatia is actively promoted.
Our Team
Our team brings together early-career researchers, postdoctoral scientists, and technical staff from diverse backgrounds including biology, biotechnology, medicine, and pharmacy. We work collaboratively, creatively, and solution-oriented - interdisciplinary within the faculty, in close cooperation with regional industry and research partners, and embedded in an international network.
Through our research, we aim to elucidate fundamental biological mechanisms while simultaneously developing concrete, sustainable, and innovative therapeutic concepts with long-term translational potential toward clinical application.

f.l.t.r.: Leo, Robby, Muazzez, Johanna, Erik, Marie, Nadine, Rizka, Patrick