Research focus
Our group focuses on the application of patient-specific induced pluripotent stem cells (iPS cells) to study developmental processes and for the establishment of in vitro disease models. We use these models to study the molecular mechanisms underlying physiological developmental processes and pathophysiological states. Patient-specific iPSCs can be reprogrammed from normal body cells (e.g. skin cells, blood cells) by overexpressing specific transcription factors to induce two important characteristics of embryonic stem cells. These include the ability of self-renewal as well as the differentiation potential (pluripotency). Self-renewal is a special form of proliferation in which both daughter cells receive the same stem cell abilities, with pluripotency enabling differentiation into specialized body cells (e.g. heart cells, nerve cells, hepatocytes, muscle cells). Patient-specific iPS cell models represent models system which are very relevant in various areas of basic research. Finally, we aim to use the new insights gained from our basic biomedical research to develop improved therapeutics and new clinical treatment approaches.
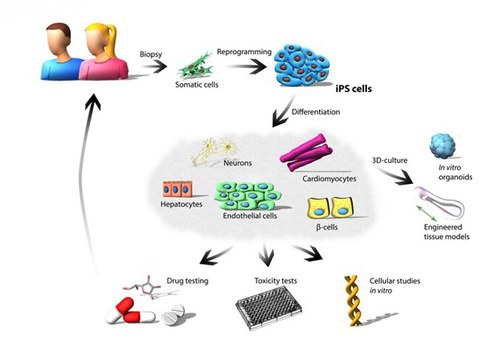
Figure 1: Application of human iPS cells. Reprogramming of somatic cells into patient-specific iPS cells represents a valuable tool for modeling and studying diseases in vitro. Human somatic cells are reprogrammed by overexpression of the four Yamanaka factors (OCT4, SOX2, KLF4 and c-MYC). The generated iPS cells can differentiate into various cell types and enable in vitro disease modelling, toxicity studies and preclinical drug screenings.
Research topics
I. Modeling developmental processes in vitro
An important application of iPS cells in basic research is the analysis of the complex developmental processes during early embryogenesis. Since in vitro differentiation of iPSCs can recapitulate the processes of embryonic development, we use this approach to study the developmental processes of early embryogenesis at the cellular and molecular level. Of particular interest is the regulation of certain key molecules during early development of the heart and pancreas.
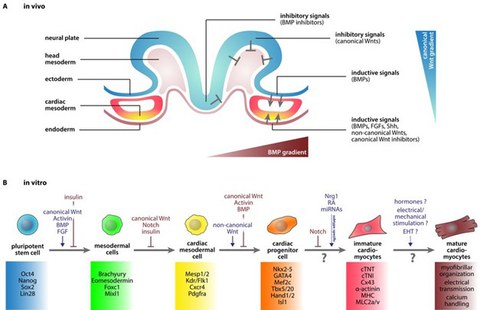
Figure 2: In vivo and in vitro cardiogenesis. (A) Scheme of early embryogenesis displaying the signaling patterns during early heart development in vivo. (B) Cardiac in vitro differentiation of iPS cells into functional cardiomyocytes. (Cyganek et al., 2013).
To further understand and analyze the role of specific genes during heart development or in specific disease states, we use CRISPR/Cas9-based gene editing in our iPS cells. For example, we have demonstrated that knockout of the ryanodine receptor type 2 (RYR2) does not impair the differentiation of cardiomyocytes from human iPSCs, despite this calcium channel is critical for excitation-contact coupling. Biochemical and functional analysis revealed that the loss of RYR2 is compensated by an increased activity of the IP3R (inositol-1,4,5-triphosphate receptor) (Luo et al. 2020).
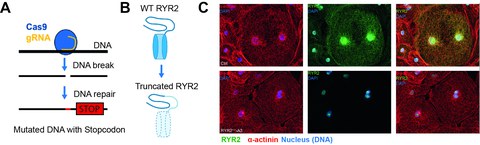
Figure 3: Knockout of ryanodine receptor 2 (RYR2) in iPSCs using CRISPR-Cas9 genome editing. (A) Scheme of the Cas9-mediated induction of double-strand breaks, which can lead to mutations such as insertions or deletions during endogenous DNA repair. As a consequence these lead to premature stop codon and termination of the transcription. (B, C) Inhibition of transcription of the membrane domain (B), which is essential for the biological function of RYR2 results in knockout of RYR2 at the protein level, which was detected by immunofluorescence staining (C) (Luo et al., 2020).
II. iPS-based disease modeling
Genome-wide association studies revealed that many human diseases are caused by monogenic defects in genes encoding structural or regulatory proteins. These diseases resulting from genetic defects are extremely relevant in the field of cardiovascular research, which is the focus of our research. In our working group, we have established various iPS cell lines from patients suffering from genetic heart diseases (e.g. catecholaminergic polymorphic ventricular tachycardia, Brugada syndrome, long QT syndrome, Barth syndrome, Vici syndrome and dilated cardiomyopathy). Since the iPS cells and the differentiated cells obtained from the iPS cells harbor the identical gene profile as the respective patients, we use patient-specific iPS cells to recapitulate and to examine aspects which occurred in the respective patient in the clinic in our cell culture dish. By using these patient-specific iPS cells and differentiated heart muscle cells, we are trying to get a deeper insight into the genetically determined pathophysiological mechanisms involved in the development of heart failure and cardiac arrhythmias in these patients. For example, we were able to show that cardiomyocytes differentiated from iPS cells (iPSC-CMs) from a patient with Brugada syndrome (with mutation p.S1812X in the sodium channel gene SCN5A) recapitulate the electrophysiological dysfunction observed in the patient in vitro, such as a disturbed depolarization and slowed conduction speed. We also use CRISPR/Cas9-based gene editing to characterize the influence of a specific mutation on the on cell function. With this approach, we are able to correct a mutated gene with the aim to restore the normal phenotype. Correcting the defective target gene is also important to ensure that the mutation in the patient is actually the cause of the disease and thus important for the molecular diagnosis.
III. Validation of new drug targets
In the past two decades, many different animal models were established in order to understand the origin, development and progression of diseases. However, animal models often only inadequately reflect the mechanisms of human disease states, due to the profound genetic and physiological differences in comparison to humans. In contrast, studies to validate therapeutic targets directly in humans are only possible to a very limited extent and furthermore, the availability of human tissue/cell material is often limited. Here, our iPS cell-based model systems enable us to perform biochemical and molecular studies using engineered human tissue and to elucidate the signaling pathways and key molecules involved in human physiology. In this research topic, we are working on different projects that focus on the role of potential drug targets in the context of diabetic cardiomyopathy or metabolic heart diseases, ischemia-reperfusion damage or the signaling pathways of cyclic nucleotides. In this way, we aim to validate new drug targets and to gain important insights into the mode of action of potential new active substances.
IV. Establishment of different iPSC-based tissue models for in vitro drug testing
About 30% of the new drug candidates are abandoned in clinical trials due to lack of effectiveness. A major reason for this is that the preclinical studies in drug development are heavily based on animal models. For example, a number of drugs have been developed which have shown therapeutic efficacy in animal models of amyotrophic lateral sclerosis but have been ineffective when tested in patients. This highlights the need to develop reliable disease models based on human cells for preclinical drug validation. Thus, cell culture models and organ-like tissues (organoids) based on human iPS cells are promising approaches to bridge the gap between classic animal model-based preclinical development and clinical reality. In our lab, we have established protocols for the directed differentiation of iPS cells into somatic cells including cardiomyocytes, neurons and hepatocytes. In addition, a variety of human organoid culture tissues (including cardiovascular, cerebral, and hepatic organoids) consisting of structured, three-dimensional cell aggregates are produced from patient-specific iPS cells. We will test the influence of experimental substances (e.g. antiarrhythmics) - with specific as well as pleiotropic effects - on the properties of human cardiomyocytes and in particular investigate the mechanisms how these substances lead to improved function. Therefore, our models based on patient-specific iPS cells are particularly valuable and allow us to characterize a variety of relevant pathophysiological aspects, even when the disease affects multiple organs.
Another key problem of new drug candidates in the human organism is the potential toxicity of these new substances. In addition to the 30% of drug candidates discontinued due to lack of clinical efficacy, another 30% of drugs tested in clinical trials were rejected due to safety concerns, such as cardiotoxicity and hepatotoxicity. Importantly, this happens despite at least two in vivo animal models (at least one rodent and one non-rodent) that are applied during preclinical drug development to estimate the toxicity of a new drug. Therefore, human cell models, especially cardiomyocytes and hepatocytes derived from human iPS cells, represent a unique and predictive model to investigate potential cardiotoxic and hepatotoxic effects of new pharmacological drug candidates already in the preclinical testing phase.
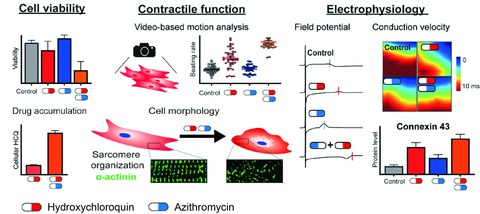
Figure 4: Characterization of cardiotoxic effects of azithromycin-hydroxychloroquine combination therapy using iPS cardiomyocytes using our wide range of methods. Scheme reflecting the variety of methods that can be applied to study disease mechanisms and adverse drug effects using iPSC-derived cardiomyocytes. In this example, our studies revealed that the negative effects of both substances on the vitality, structure and contractile and electrophysiological function of human heart muscle cells are synergistically enhanced by their combination (Li et al., 2020).
Laufende Projekte
Leptin and leptin receptor signalling in diabetic cardiomyopathy
International Research Training Group 2251: Immunological and Cellular Strategies in Metabolic Disease (ICSMD); Link: https://tu-dresden.de/med/mf/irtg2251
Contact: Prof. Dr. Kaomei Guan
Staff: Dr. Anna Strano, MSc. Irem Congur, MSc. Vanya Milanova, MSc. Fatima Kanwal Baig, MD Josie Schnarr
Leptin is a central hormone for the regulation of food intake through triggering satiety and thus limiting caloric input. Mutations in leptin or the leptin receptor lead to severe forms of obesity, that are already apparent in childhood. Resistance to leptin is also common in obese or overweight patients as well as in patients type 2 diabetes, in whom reduced insulin sensitivity or insulin resistance is also relevant. These patients have a high prevalence for cardiac complications and the development of heart failure. This form of metabolic heart disease establishing in these patients is referred to as diabetic cardiomyopathy. Our project aims to focus on the role of leptin and on the consequence of a disrupted leptin signaling cascade (using CRISPR/Cas9) in the development of diabetic cardiomyopathy using induced pluripotent stem cell-differentiated human cardiomyocytes.
„MultiMOD“ - Smart material-based circuits for the multimodal information space
ESF/ESF Plus Projekt (Speaker: Prof. Dr. Andreas Richter),
Link: https://tu-dresden.de/ing/elektrotechnik/ihm/ms/forschung
Contact: Prof. Dr. Kaomei Guan
Staff: Dr. Mario Schubert, Dr. Xiaojing Luo, Dr. Anna Strano
The junior research group MultiMOD aims to establish the basis of a circuit and information processing concept based on smart materials. This enables the direct processing of various physical, chemical and biological information from our environment without the need for electronic components. These circuits allow many more functions to be integrated at the chip level, including information processing, power supply, sensors and actuators. They are extremely energy efficient, require only a fraction of the resources used in traditional microelectronic systems and can be recycled highly efficiently. The junior research group focuses on three specific applications in life sciences which would greatly benefit from the capabilities of these multimodal circuits: multi-organ-on-chip systems with complex chemical-biological regulatory mechanisms, multi-modal circuits for early cancer detection, and micro-robotic systems with true-to-life capabilities for use as a surgical robot.
Stem cell-based in vitro platform for differentiated precision diagnostics and therapy development for heart disease (CardioEpiX)
Funded by Sächsischer Aufbaubank (SAB)
Contact: Prof. Dr. Kaomei Guan
Staff: Dr. Wener Li, Dr. Mario Schubert
Atrial fibrillation (AF) is a major cause of stroke, myocardial infarction and heart failure, associated with significant morbidity and mortality worldwide. To address the global magnitude of AF, it is necessary to develop new tools to serve the demands in terms of differential early detection, accurate classification and therapy development.
The CardioEpiX project addresses this issue by providing a solution for precise patient- and clinical picture-differentiated in vitro diagnosis of different forms of AF. The consortium consists of the scientific company Sciospec Scientific Instruments GmbH and two research institutions in Saxony, TU Dresden and University of Leipzig.
At TU Dresden, we focus on the establishment and characterization of different, clinically relevant forms of AF using in vitro phenotyping of patient-specific induced pluripotent stem cell-derived cardiomyocytes. Moreover, we will apply the newly developed platform to study drug efficacy and to perform risk stratification of drugs using the AF models.
Technology Platform for Modular Micro-Physiological Systems (TECH-MPS)
Funded by the Sächsische Aufbaubank (SAB), Project duration 15.08.2025-31.12.2027
Animal studies in drug development pose significant ethical and practical challenges. A central issue represents the limited translatability of results from animal studies to humans. Here, the application of human cell models in combination with micro-physiological systems (MPS) offers a sustainable approachto improve drug development by increasing efficiency, reducing costs, and minimizing animal testing.
The goal of the TECH-MPS project is to develop a comprehensive technological platform that provides flexible, modular MPS while enabling seamless integration and analysis of data from manufacturing, preparation of cells and tissue models, their culture within the MPS, and experimentally generated datasets.
In our group, we focus on the generation of advanced human cardiac cell models to establish an MPS-based model of myocardial infarction (MI). This model served as a reference application of the MPS platform and will be employed to investigate mechanisms that reduce cardiac muscle damage and to test potential drug candidates.
Consortium:
Interaction of cardiomyocytes and cardiac fibroblasts in heart development and pathophysiological processes
Formerly supported by MeDDrive Grant der TU Dresden (to M. Schubert)
Link
Contact: Dr. Mario Schubert, Prof. Dr. Kaomei Guan
Staff: MD Wilhelm Wenke, MD Paul Josef Conrad Beck
Maturation of iPSC-derived cardiomyocytes in 2D and 3D-models for drug testing and disease modeling
Formerly supported by MeDDrive Start der TU Dresden (to Dr. M. Schubert)
Link
Contact: Dr. Mario Schubert, Prof. Dr. Kaomei Guan
Staff: MD Paul Josef Conrad Beck, MSc. Oliver Gamm, MSc. Yuliya Dzekktsiarova, MD Paul Geppert