Publications
In pride of place - see our Cover Gallery Ersatz and list of No-OTS Papers
Web publists via Google Scholar, ORCID, or PubMed
Paper explainer threads on BlueSky; more through the Bsky #ChemBio community
Pub list: jump to: 2025, 2024, 2023, 2022, 2021, 2020...
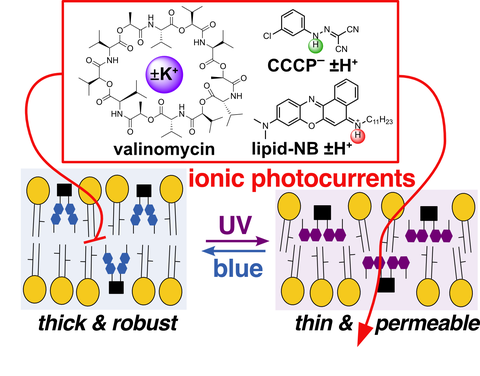
62. J Pfeffermann, R Yadav, T Glasnov, O Thorn-Seshold, P Pohl*;
Optical control of carrier-mediated ion transport by photoswitchable lipids
Nanoscale 2026 (qjbz)
Most photocontrol work so far aimed at single reagents that photoswitch their (a) specific pharmacology, for cell biology; or (b) general physicochemical properties, for biophysics. We develop a new hybrid:
(c) photocontrolling general membrane permeability switches in the presence of specific but non-photoresponsive ion carriers, to drive powerful yet specific ion photocurrents. This two-module approach also offers the perspective to control membrane transit of diverse ions and molecules into any cell type, without genetic engineering. Bsky thread here.
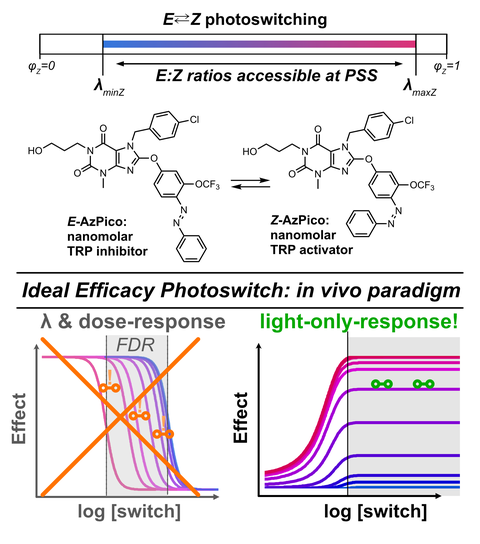
61* M Müller, K Niemeyer, NK Ojha, SA Porav, D Vinayagam, N Urban, F Büchau, K Oleinikov, M Makke, CC Bauer, AV Johnson, SP Muench, F Zufall, D Bruns, Y Schwarz, S Raunser, T Leinders-Zufall, RS Bon, M Schaefer*, O Thorn-Seshold*;
Ideal efficacy photoswitches for TRPC4/5 channels harness high potency for spatiotemporally-resolved control of TRPC function in live tissues
Nature Chemical Biology 2026 (qndx)
Here we solved a previously-unrealised systematic problem that had blocked nearly all photoswitchable reagents from in vivo utility. To date, nearly all reagent designs were affinity photoswitches for intensiometric photocontrol. These are easy to create by classic drug design, but are only effective when drug concentrations are fixed. This is fine for publishable results in 2D cell culture, but upon transition to in vivo work, ADME-PK makes them useless. This had left photopharmacology unable to compete with optogenetics, whose in vivo applicability is its greatest strength.
● We now invented the concepts of ideal efficacy photoswitches for ratiometric chromocontrol over protein activity, to solve this translational problem. Ideal efficacy switches are just as effective over variable concentrations in vivo, as under fixed conditions in vitro. This brings a massive upgrade to the real-world applicability of high-precision photopharmaceuticals, that can finally make them more powerful than optogenetics (broader in vivo scope, endogenous modulation, etc).
This paper shows how to rationally design this new class of reagents, develops the first ever photoprobes for the multifunctional signaling ion channels TRPC4 and TRPC5, and applies them across scales from cryoEM (with 4 structures that indicate the structural basis of TRPC4/5 allosteric control), to cell biology, tissue slices, and in live organs, where our photoswitches now show the role of TRPC4 in digestive neuromuscular coupling.
Tutorial style walkthrough here as a BlueSky thread.
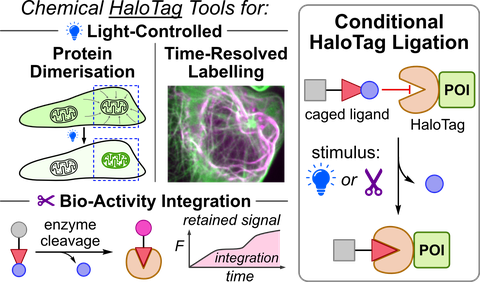
60* P Mauker, C Zecha, L Dessen-Weissenhorn, L Roman-Albasini, J Meiring, J Brandmeier, D Beckmann, JP Prohaska, M Reynders, YL Li, K Mrug, H von Schwerin, N Veprek, J Gaisbauer, L Dehmelt, P Nalbant, J Zenker, A Akhmanova, M Kerschensteiner, A Harbauer, J Thorn-Seshold, O Thorn-Seshold*;
Logic-gating the HaloTag system with Conditional-Halo-ligator 'CHalo' reagents
BioRxiv 2025 (doi.org/p68c)
HaloTag proteins spontaneously ligate to chemical reagents that bear a linear haloalkane motif ("CA" or "HTL" ligands). We now introduce the conditional CHalo motif, which ligates to HaloTag only after it is uncaged by light, enzyme activity, or chemical reaction. We show that (1) photo-triggered CHalo fluorogenic reagents allow spatiotemporally-specific labeling; (2) photo-triggered CHalo heterodimerisers can photocontrol protein recruitment; (3) biochemically-triggered CHalo reagents can durably record diverse enzyme activities or small molecule reactants. Since multiple CHalo reagents can be recorded onto a single type of HaloTag, it should prove possible to use CHalos for cell-resolved ratiometric quantitative imaging of multiple enzyme activities in parallel, e.g. to uncover (de)coupled enzyme activities in systems biology. CHalo thus permits manifold extensions to the HaloTag technology.
59* O Thorn-Seshold*, P Mauker;
Compound, composition, kit and their use for protein labelling
Patent Application 2025, LU602798
Here, we disclose the cageable HaloTag-reactive motif "CHalo", which ligates to HaloTag only after uncaging by a stimulus e.g. from light, enzyme activity, [bioorthogonal] chemical reaction, etc. Caged-CHalo reagents can support diverse logic-gated uses of HaloTag proteins: from passively recording multiple stimuli in parallel, to externally triggered recruitment or functionalisation of HaloTag fusion proteins.
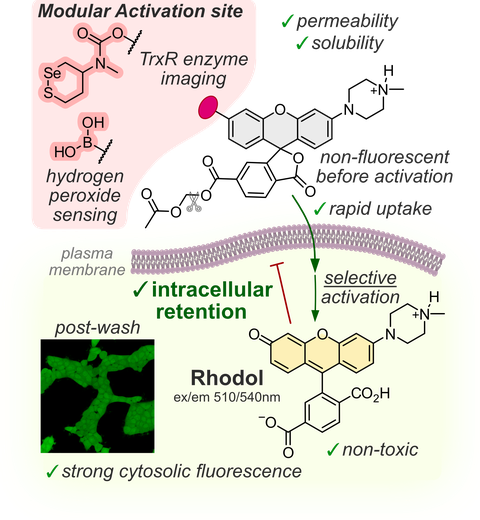
58* P Mauker, L Dessen Weissenhorn, C Zecha, N Veprek, J Brandmeier, D Beckmann, A Kitowski, T Kernmayr, J Thorn-Seshold, M Kerschensteiner, O Thorn-Seshold*;
Cellularly-Retained Fluorogenic Probes for Sensitive Cell-Resolved Bioactivity Imaging
Chemical Science 2025 (doi.org/p9ht)
Fluorogenic enzyme turnover probes for live cells must be membrane-permeable; but that often means that their fluorophore products are similarly permeable: so signal is lost from the activating cell, sabotaging both cell-by-cell resolution and sensitivity for low-turnover processes. Here, we develop a general design for high-quality fluorogenic activity probes that efficiently generate a cell-retained, bright fluorescent soluble product. The rhodol Trappable Green (TraG) balances key needs for signal integration (rapid cell entry, but effective product retention, across a variety of cell lines) and it is easily adaptable for many biochemical targets (here: GSH, TrxR, and H2O2). This rugged scaffold can now be elaborated for a range of cell-retained enzyme activity probes, that enable more accurate cell-resolved imaging as well as higher-sensitivity integration of low-turnover processes.
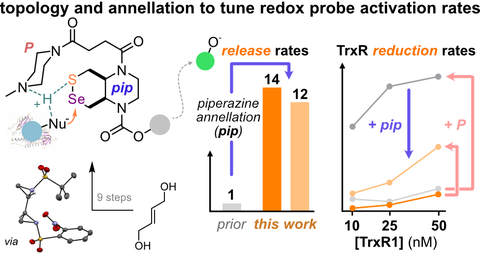
57* L Zeisel*, L Dessen Weissenhorn, K Scholzen, A Madabeni, L Orian, E Arner, O Thorn-Seshold*;
Bicyclic Selenenyl Sulfides with Tuned Bioreductive Step Rates Reveal Constraints for Probes Targeting Thioredoxin Reductase
Angewandte 2025 (doi.org/ptv9)
Cyclic selenenyl sulfides are key intermediates in redox biochemistry, but are drastically understudied as a chemical class, with only ca. 15 unique scaffold structures reported (fewer even than the ~25 human selenoproteins). Here we designed the first aliphatic bicyclic selenenyl sulfide, to merge several reactivity hypotheses into a single densely-functionalised core [cyclic RSeSR', piperazine-annellated, cis fusion, 1,2-Se,N disposition]. We provide a regioselective and diastereoselective synthesis of this system, on scale, by a short sequence involving three aziridines as convenient control elements for 1,2-functionalisations. These combined features yielded RSeSR'-type redox probes that strongly resist off-target activation by monothiols yet rapidly release a cargo upon reduction, and efficiently respond to the mammalian selenoenzyme TrxR.
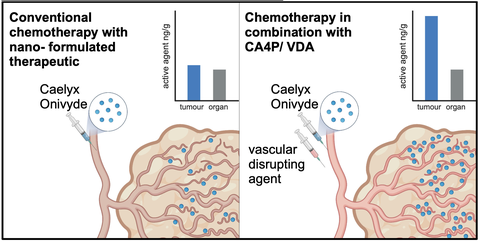
56. A Kitowski, C Heise, S Sperling, J Hotz, D Bajic, M Rubey, K Klar, G Höfner, J Vollaire, V Josserand, JL Coll, O Thorn-Seshold, P Marinković, J Thorn-Seshold*;
The delivery of nano-formulated drugs to solid tumours is selectively increased by co-application of the vascular disrupting agent CA4P
BioRxiv 2025 (doi.org/p2n3)
Improving the efficacy of known anticancer drugs, by delivering them more selectively to tumours, is a compelling alternative to searching for new chemotherapeutics. Here we present results from our GO-Bio translational preclinical project (2015-2022), showing that the intrinsic sensitivity of tumour vasculature to microtubule-depolymerising vascular disrupting agents (VDAs) can be used to enhance the tumour-selective delivery of drugs that have been formulated into liposomal nanoparticles, independent of the drug type. Co-administering a VDA together with clinically approved nano-formulations of irinotecan and doxorubicin increased tumoural accumulation rate as well as tumoural retention: reaching threefold exposure (AUC) in a syngeneic mouse model of triple-negative breast cancer. We had hoped this effect would either allow lower nanoparticle doses to deliver similarly effective tumour treatment with lower systemic side-effects, or else allow standard doses to deliver increased antitumour efficacy without raised side-effects. However, we found the enhancement was only reliable upon the first VDA co-administration, with no delivery enhancement at our VDA dosages in these mouse models in subsequent rounds, and no additional therapeutic benefit after repeated treatments. However, since vascular susceptibility to VDAs is a conserved feature across solid tumours, this approach may offer a broadly applicable cargo-independent strategy to improve nanoparticle delivery without appealing to personalised medicine, EPR, antigens or immunotyping: which may be helpful for single-dose applications, in research or for clinical diagnostics.
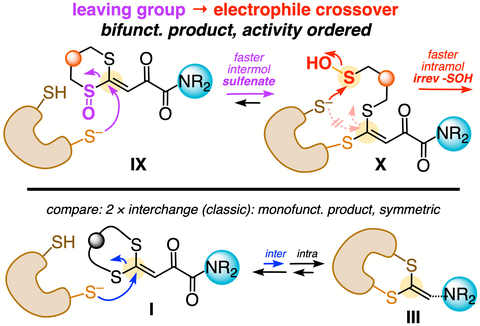
55. Y Zhang, G Renno, GJ Wørmer, AM Tamr, O Thorn-Seshold, TB Poulsen*, N Sakai, S Matile*;
Reversible Conjugate Addition in Thiol-Mediated Uptake
Helv Chim Acta 2025 (p6zk)
This reports the first concepts towards selective electrophilic labelling of vicinal dithiol proteins, in a way that does not label monothiols. Like with the dithiol-turnover probes we previously developed, we designed a 2-step reaction cascade so the first step is fully reversible, aiming that only vicinal dithiols could complete the second irreversible step; here requiring a bis(leaving group) motif to trap the bis(nucleophile) targets. Mono-S-oxidation achieves many features at once: it not only (a) accelerates the kinetics of the first (intermolecular) step, but also (b) allows a nucleophile-to-(thiol)-electrophile reactivity crossover which provides a faster second-step thiol-trapping pathway, and (c) gives a bifunctional product, which is also (d) desymmetrised to reveal which of the two cysteine residues initiated the reaction process. Since the mono-S-oxide also turned out to be (e) the most active enhancer of thiol-mediated uptake ever seen, this work also provides an exciting viewpoint on how vicinal dithiol type oxidoreductases probably play key, but still not yet elucidated, upstream and/or direct roles in cell-surface redox biochemistry and control the cellular import of reversibly electrophilic Michael acceptor drugs.
54. A Madabeni, L Zeisel, O Thorn-Seshold, L Orian*;
Selenium Nucleophilicity and Electrophilicity in the Intra- and Intermolecular SN2 Reactions of Selenenyl Sulfide Probes
Chem Eur J 2025 (doi.org/n3vx)
Thiol-disulfide exchange reactions are fundamental to life, e.g. in thiol redox, protein refolding, and thiol-mediated cellular uptake. Exchanges of selenenyl sulfides (RSe–SR' + R''SH ⇆ RSe–SR'' + R'SH) are far less understood, even though more than half of the selenoproteome is devoted to this reaction type. Here, we report the first study on the SN2@S and SN2@Se reactions of cyclic and linear RSe–SR' species. This (a) gives a comprehensive overview of their kinetic and thermodynamic differences vs the better-known disulfides and diselenides, then (b) maps the mechanism of cyclic RSe–SR' probe reactivity in the thiol-rich cellular environment (paper 32). These analyses support that the intramolecular SN2@Se back-reaction which restores cyclic RSe–SR' probe integrity after monothiol addition is the main protective mechanism enabling them to function in live cells: a quantitative rationalisation for the TrxR-specificity we reported several years ago.
53. A Baptist; L Guericke; P Mauker; O Thorn-Seshold; A Heuer-Jungemann*;
Customizable silicification of DNA origami nanostructures
BioRxiv 2025 (qkjm)
Silicifying organic nanostructures can greatly enhance their mechanical and chemical durability. Until now, silica coatings have generally served as a protective layer or as the base for further deposition of inorganic materials, but have not carried additional functionality. Here, we synthesise fluorophore-or disulfide-appended orthosilicate dopants and use them to grow fluorescent or redox-responsive silica shells around DNA origamis.
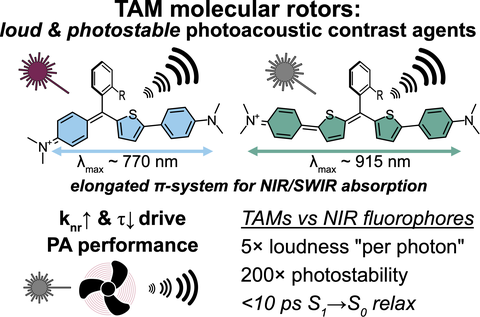
52* M Müller*, A Valavalkar, V Gujrati, JP Prohaska, D Shelar, M Kaltenegger, B Dietzek-Ivanšić, V Ntziachristos, O Thorn-Seshold*;
Molecular rotors are loud, highly photostable, NIR/SWIR- active molecular optoacoustic contrast agents
ChemRxiv 2024 (doi.org/ntpt)
Photoacoustic imaging agents are usually high-ε NIR-absorbing basis structures (developed for fluorophores) that later have their fluorescence quenched. We argue for a different conceptual approach centred on the absolute rate of nonradiative decay (paper 49); hence rewriting the design strategy: first choose a scaffold for ultrafast decay, and only later tune ε & λmax. Here we introduce the first NIR/SWIR triarylmethane PA agents (a non-fluorophore chemical space), showing how molecular rotors can unlock quantitative longitudinal PA: a rational, mechanism-based design to improve imaging dye performance.
51* O Thorn-Seshold*, V Glembockyte, B Baumgartner, A Wiegand;
Stabilised Fluorophores, compositions, methods of preparation, conjugates thereof, and methods of use
US Patent Application 2024, 18/737,536
Under high-intensity imaging, even fluorophores with low intersystem crossing yields will enter triplet states. These "dark" (non-signal-generating), long-lived, and chemically reactive triplets are the primary cause of both fluorophore photobleaching and biological photodamage. Here we disclose a general method to depopulate triplets on the fly, to convert known fluorophores into high-brightness, long-lived, photoresistant and biologically innocent imaging agents.
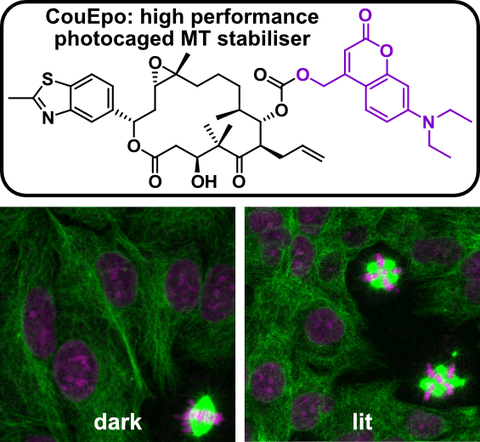
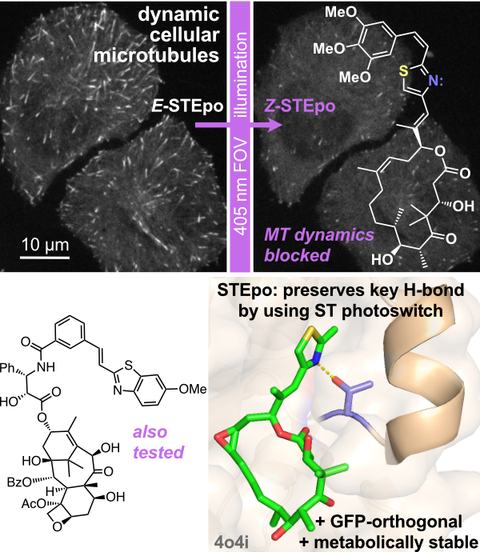
50* C Schmitt, P Mauker, N Vepřek, C Gierse, JCM Meiring, J Kuch, A Akhmanova, L Dehmelt, O Thorn-Seshold*;
A Photocaged Microtubule-Stabilising Epothilone allows Spatiotemporal Control of Cytoskeletal Dynamics.
Angewandte Chemie 2024, e202410169 (m6qr)
Cytoskeleton research requires high-precision tools to locally manipulate the dynamics and organisation of cytoskeletal proteins. Yet, to date, no photoresponsive tool could unite the features needed for a practical, high-precision microtubule-stabilising reagent: i.e., (1) inactive when not lit; (2) efficient and clean photoconversion to a high potency drug; (3) good solubility at the working concentration; (4) efficient synthetic access. We present CouEpo, the first photocaged epothilone, which is a high-performance tool compound for photocontrolling microtubule dynamics in live cells on the micron scale. It can now support high-precision research into many microtubule-associated processes, from biophysics to transport, cell motility, and neuronal physiology.
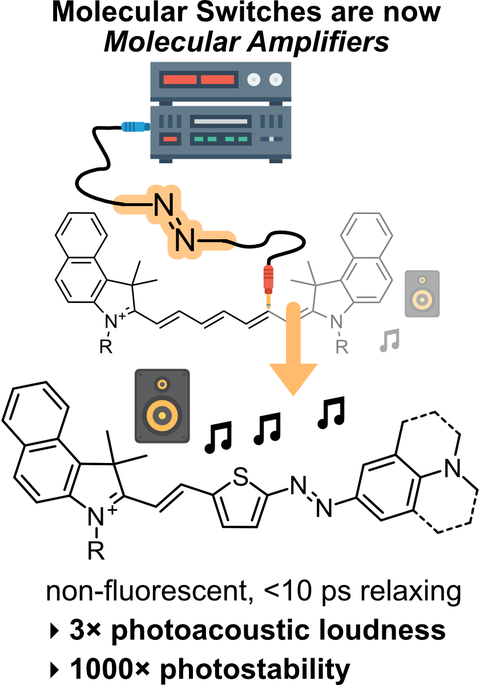
49* M Müller, N Liu, V Gujrati, A Valavalkar, S Hartmann, A Telek, B Dietzek-Ivanšić, A Hartschuh, V Ntziachristos, O Thorn-Seshold*;
Merged molecular switches excel as optoacoustic dyes: azobenzene-cyanines are loud and photostable NIR imaging agents.
Angewandte Chemie 2024 (VIP paper), e202405636 (mzgt)
Optoacoustic or Photoacoustic imaging can offer µm-resolution noninvasive imaging in biology, much deeper (>cm) than fluorescence. However, current NIR-absorbing small molecule contrast agents are not loud and photostable enough: most are simply repurposed fluorophores, with photoinstability or phototoxicity problems in optoacoustic imaging due to their slow S1→S0 relaxation.
We now rationally optimise these old dyes for optoacoustics, reaching ultrafast S1→S0 rates (<10 ps, >100-fold faster) just by merging them with molecular switches: giving outstanding improvements of signal longevity (>2000× photostability) and signal loudness (>3× even at time zero). This novel, simple strategy can increase the spatial resolution and quantitative response of longitudinal photoacoustic imaging. The world of molecular switches has much to contribute in imaging; unleashing the potential of optoacoustics is just the beginning.
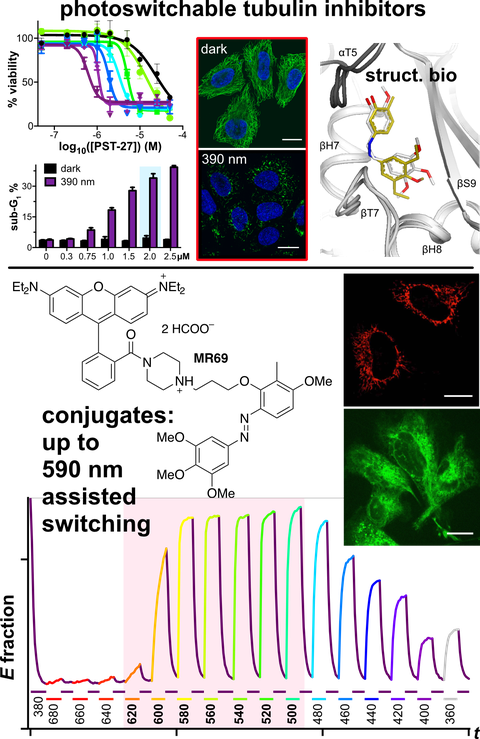
48* M Reynders, M Garścia, A Müller-Deku, M Wranik, K Krauskopf, L de la Osa de la Rosa, K Schaffer, A Jötten, A Rode, V Stierle, Y Kraus, B Baumgartner, A Ali, A Bubeneck, T Seal, MO Steinmetz, P Paulitschke, O Thorn-Seshold*;
A photo-SAR study of photoswitchable azobenzene tubulin-inhibiting antimitotics identifying a general method for near-quantitative photocontrol.
Chemical Science 2024, 12301-12309 (m6g2)
Photoswitchable combretastatin A4 azologues (photostatins, "PSTs") were previously developed to optically control microtubule dynamics in living systems. Now, (1) we used large-field-of-view, long-term photoswitching/microscopy to fill the gap between their seconds- and hours-scale photopharmaceutical bioactivity; and (2) we tested a panel of PST structural variations for photo-SAR to tune photoresponse and bioactivity, for GFP/YFP-compatible analogues. These results guide new design and applications for photoswitchable microtubule inhibitors. (3) We also attached fluorescent reporter "antennas", which greatly enhanced the long-wavelength single-photon photoisomerisation of azobenzenes by a hitherto un-explored mechanism (see e.g. papers 42 & 47), which will drive general progress towards near-quantitative long-wavelength photoswitching of photopharmaceuticals in biology.
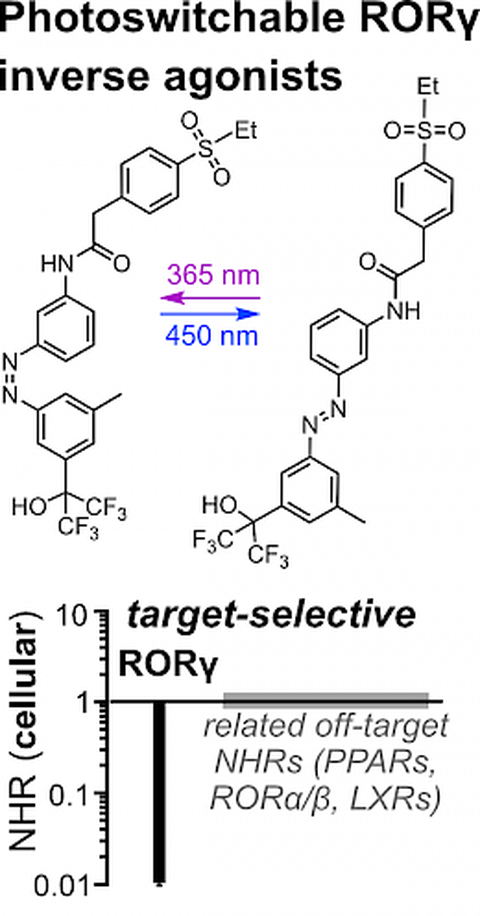
47* M Reynders*, S Willems, J Marschner, T Wein, D Merk*, O Thorn-Seshold*;
A High-Quality Photoswitchable Probe that Selectively and Potently Regulates the Transcription Factor RORγ.
Angewandte Chemie 2024, e202410139 (ngvr)
Retinoic acid receptor-related orphan receptor γ (RORγ) is a circadian regulator with a cyclic temporal role, as well as a spatially-localised target for experimental therapeutics in inflammation and immunity. To make tools to study RORγ biology with the spatial and temporal precision its biology requires, we designed photoswitchable RORγ inverse agonists that are a rare example of high-quality photoswitchable probes: combining nanomolar potency that can be photoswitched by >10-fold in cells, with outstanding selectivity over related nuclear receptors. These photopharmaceuticals can now serve as high-precision tools to study the dynamic modulation of RORγ in signaling pathways and in inflammatory disorders.
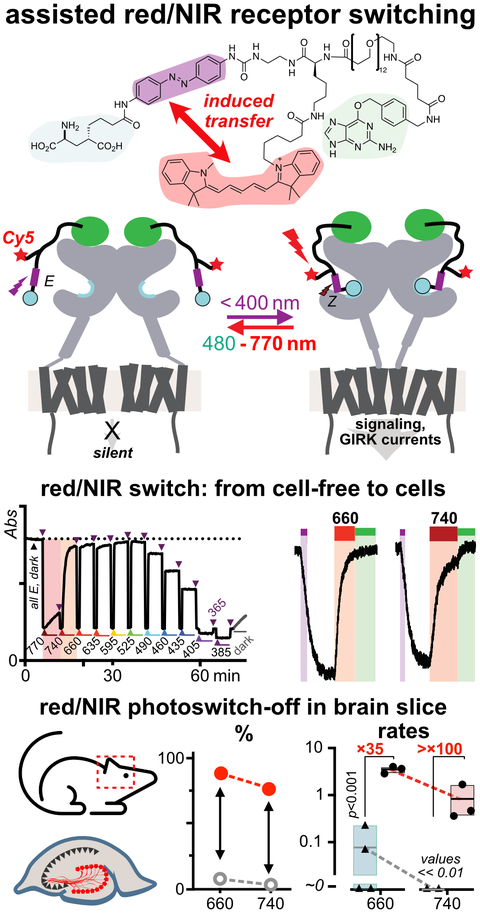
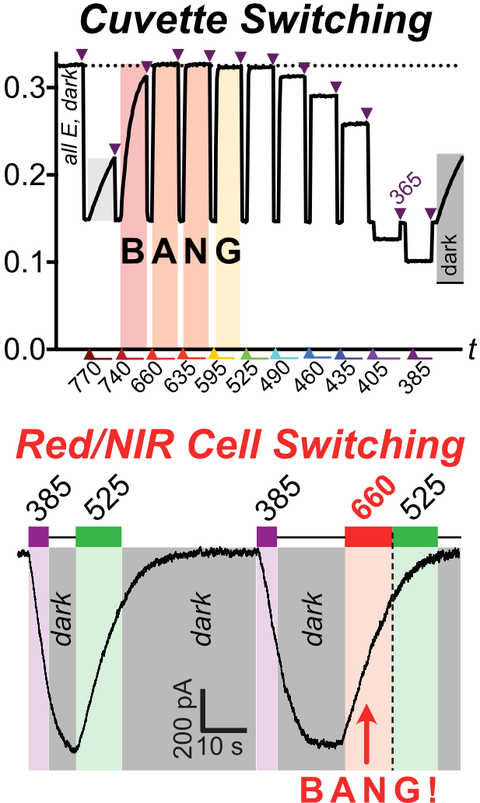
46* B Baumgartner, V Glembockyte, A Gonzalez-Hernandez, A Valavalkar, R Mayer, L Fillbrook, A Müller-Deku, J Zhang, F Steiner, A Wiegand, C Gross, M Reynders, H Munguba, A Arefin, A Ofial, J Beves, T Lohmüller, B Dietzek-Ivansic, J Broichhagen, P Tinnefeld, J Levitz, O Thorn-Seshold*;
A General Method for Near-Infrared Photoswitching in Biology, Demonstrated by the >700 nm Photocontrol of GPCR Activity in Brain Slices.
ChemRxiv 2024 (doi.org/mtnw)
Azobenzene molecular switches are widely used to photocontrol cellular activity and material properties by E⇆Z photoswitching. However, since this photoswitching is incomplete, their dynamic range of property control is often small; and as they cannot be operated with red/NIR light, they are usually not applicable in deep tissue in vivo. Here, we pioneer a very general method for efficient single-photon >700 nm photocontrol of azobenzenes: conditional energy transfer from red/NIR chromophore auxiliaries to bioactive azobenzenes, driving bulk Z→E isomerisation to even >95% completeness with photon-efficiency that can be even higher than for direct azobenzene E→Z isomerisation in the UV region. The transfer reagents are biocompatible and photostable, and crucially, their performance properties are intrinsic: i.e. will perform identically at any dilution, and not be affected by biodistribution. We show that these dyads can be created straightforwardly from most azobenzenes, with most auxiliary chromophores, without tricky re-design or re-optimisation. After outlining some rules of auxiliary-based photoswitching, which can guide its broader adoption, we conclude by validating the method for single-far-red-photon photocontrol of glutamate receptor activity in live acute brain slices. (See also papers 45 & 48).
45* B Baumgartner, V Glembockyte, RJ Mayer, AJ Gonzalez-Hernandez, RO Kindler, A Valavalkar, AJ Wiegand, A Müller-Deku, L Grubert, F Steiner, C Gross, M Reynders, V Grenier, J Broichhagen, S Hecht, P Tinnefeld, AR Ofial, B Dietzek-Ivanšic, J Levitz,
O Thorn-Seshold*;
Azobenzenes can achieve near-infrared photocontrol in biological systems, with quantitative Z→E photoisomerization, via singlet manifold photoredox.
ChemRxiv 2023 (doi.org/ks9v)
Despite two decades of optogenetics and photopharmacology, we still cannot photoswitch bioactivity with NIR light: so we are blocked from true in vivo applications of these powerful and diverse reagents.
Here we launch the first general solution for NIR-photoswitching bioactivity. We show how to retrofit the hundreds of existing photopharmaceuticals that have been applied with UV/blue light in cell culture, to make them NIR-operated for in vivo work, while inheriting unprecedented photon-efficiency and complete Z→E photoswitchability in the biological transparency window: thus greatly improving their performance and practical applicability. The world of molecular switches has so much to contribute to in vivo biology; this "first" of "NIR-shifting" is just the beginning (see papers 46, 48, and more to come).
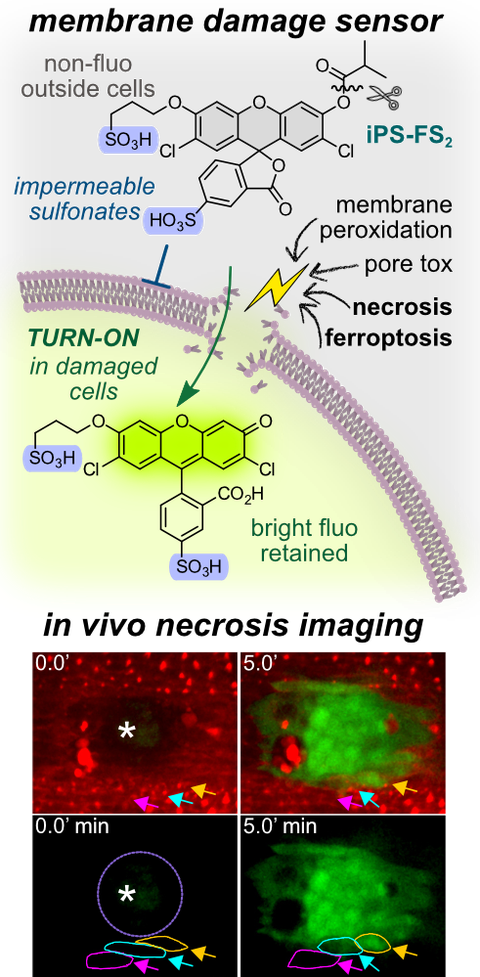
44* P Mauker, D Beckmann, A Kitowski, C Heise, C Wientjens, AJ Davidson, S Wanderoy, G Fabre, A Harbauer, W Wood, C Wilhelm, J Thorn-Seshold, T Misgeld, M Kerschensteiner, O Thorn-Seshold*;
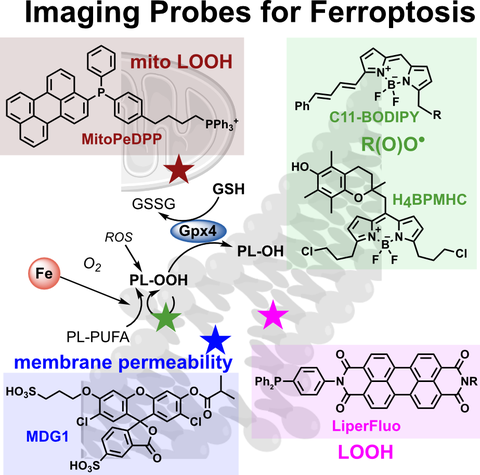
Fluorogenic chemical probes for wash-free imaging of cell membrane damage in ferroptosis, necrosis, and axon injury.
Journal of the American Chemical Society 2024, 11072-11082 (mrgt) (pp)
Selectively labelling cells with damaged membranes is needed in contexts as simple as identifying dead cells in culture, or as complex as imaging membrane barrier functionality in vivo during ferroptosis or necrosis. To do this more powerfully, we require tools that no longer need removal of extracellular signal before imaging.
We now developed in vivo-competent disulfonated fluorogenic probes which reveal cells whose membrane integrity has been damaged by e.g. ferroptosis, necrosis, or bacterial toxins. We identify a priviliged scaffold that can be modularly adapted for a range of fluorogenic probes for damaged cells, or molecular imaging of cell-surface enzymes in healthy cells. The insights gained from these probes should also be translatable to damage-targeted prodrugs, for selective therapy of membrane-compromised cell populations e.g. in neurodegeneration.
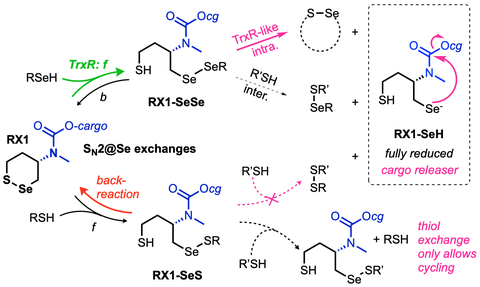
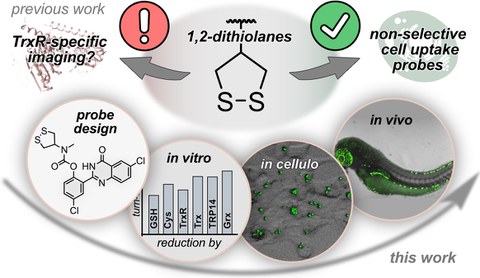
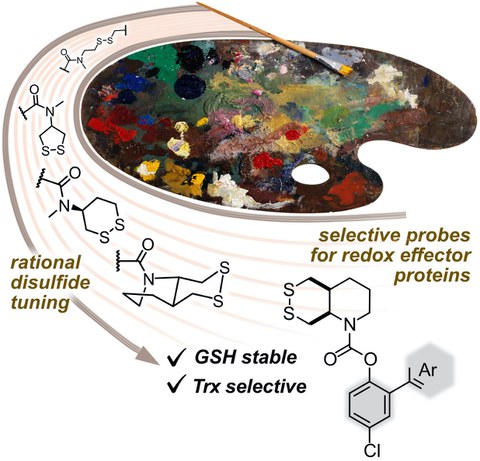
43* L Zeisel#, J Felber#, K Scholzen, C Schmitt, A Wiegand, L Komissarov, E Arnér, O Thorn-Seshold*;
Piperazine-fused cyclic disulfides: high-performance bioreduction-activated cores for bifunctional probes and reagents.
Journal of the American Chemical Society 2024, 5204-5214 (mjhj) (pp)
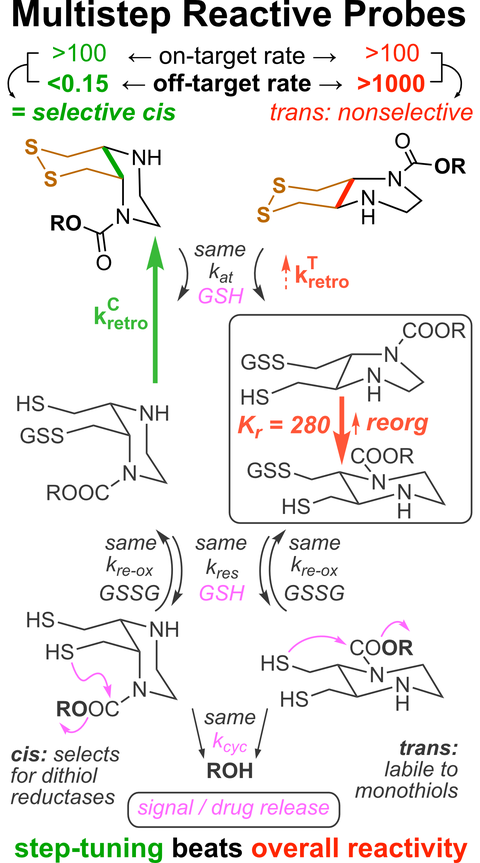
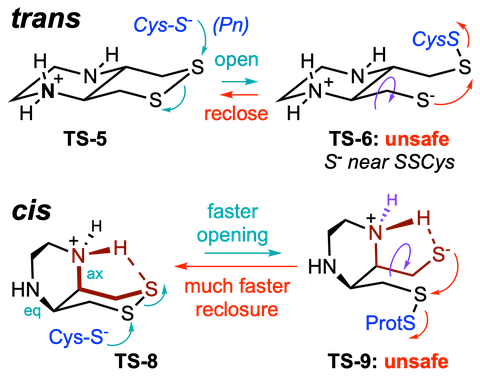
"Concept summary": The classic limitation of single-step reactive probes is that increasing reactivity comes at the cost of decreased selectivity. We now show that multi-step cascades can tune selectivity and reactivity independently: so, when the rate-limiting steps are different between on-target and off-target pathways, chemical design addressing these steps separately can increase both selectivity and reactivity, to access unprecedently powerful probes for biological systems.
"User summary": there were no cellularly-effective reagents to selectively address dithiol reductases like Trxs and Grxs. We developed piperazine-fused six-membered-cyclic disulfides to fill this gap. These probes are >100-fold faster redox-activated than any previously known; are good substrates for thioredoxins; and can selectively and effectively report Trx activity in live cells. Late-stage diversifications can apply these motifs not just in redox probes and prodrugs, but also for solid phase synthesis or antibody-drug conjugates. This work thus establishes tuned dichalcogenides as flexible and effective bioreductive triggers for cellular redox biology.
42* O Thorn-Seshold*, J Felber*, L Zeisel*;
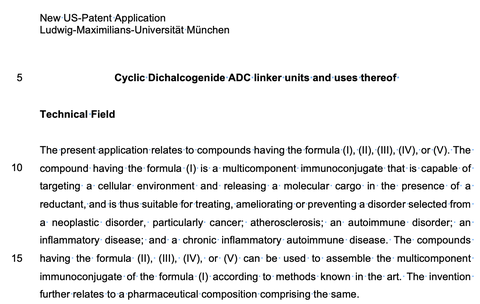
Cyclic Dichalcogenide ADC Linker Units and Uses Thereof.
US Patent Application 2024, 18/587313
A modular drug delivery system using intracellular dichalcogenide redox.
41* F Coelho, L Zeisel, O Thorn-Seshold*, S Matile*;
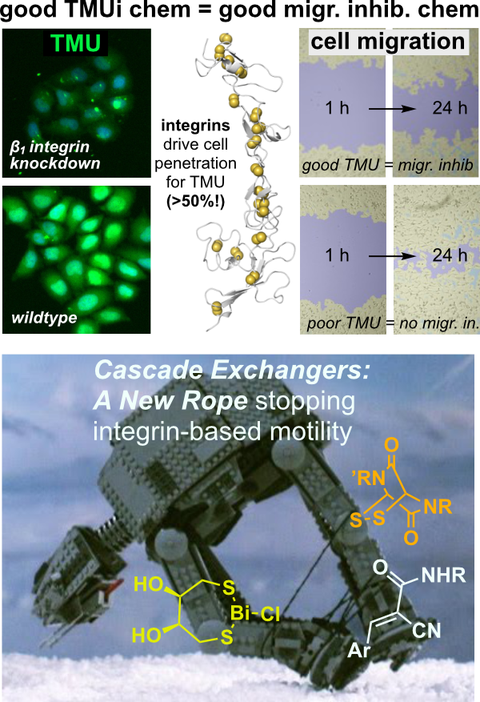
Selenium-Centered Cascade Exchangers and Conformational Control Unlock Unique Patterns of Thiol-Mediated Cellular Uptake
Chemistry Europe 2024, e202400032 (ngh8) (ChemView highlight)
Introducing cyclic selenenylsulfides (SeS) as a new dynamic-covalent electrophile motif that can reversibly engage cell-surface thiols to increase thiol-mediated cargo uptake (TMU) into cells. We show how remote ammoniums and conformational effects can tune uptake activity, and show that SeS are unique in that they actually increase the TMU of co-applied Michael acceptors. This also underscores the complex links between exofacial redox status, and real or apparent intracellular redox activity.
40 C Berndt*; coauthors incl. C Schmitt, O Thorn-Seshold, & 88 more; M Conrad*;
Ferroptosis in health and disease §6.3.1: Fluorescent Probes to Investigate Ferroptosis
Redox Biology 2024, 103211 (gtw5m4)
Ferroptosis is a non-apoptotic mode of cell death where phospholipid hydroperoxides are formed by peroxidation chain reactions, leading to plasma membrane rupture. How lipid peroxidation is initiated or prevented, and how it causes ferroptosis, are subjects of intense research; but investigating these requires solid biochemical techniques to quantify and speciate the molecular actors involved. Our section in this mega-review is a short critical review of the fluorescent probes for tracking and characterising ferroptosis-related cellular features.
39* J Felber, A Kitowski, L Zeisel, M Maier, C Heise, J Thorn-Seshold, O Thorn-Seshold*;
Cyclic dichalcogenides extend the reach of bioreductive prodrugs to harness the thioredoxin system: applications to seco-duocarmycins.
ACS Central Science 2023, 763-776 (j4cq)
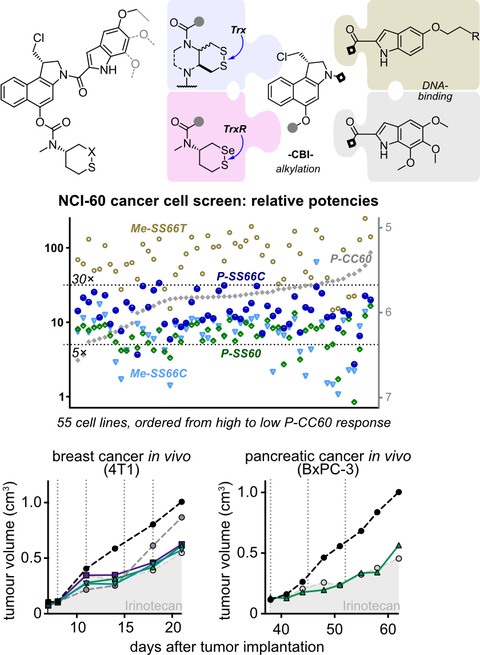
Despite their crucial roles in physiology as well as links to cancer and inflammation, cellular dithiol-type oxidoreductases such as Trx/TrxR cannot be addressed by current bioreductive prodrugs, that mainly cluster around oxidised nitrogen species. Here we harnessed artificial dichalcogenides to gate the bioactivity of a series of 10 "off-to-on" reduction-activated cytotoxic duocarmycin prodrugs. Screening across 177 cell lines with reference to hydrolytic controls let us quantify their true reduction-specific activation index. Next, a combination of good tolerability with robust antitumour efficacy was shown in vivo in mouse models of breast and pancreatic cancer: a challenge with this famously toxic cargo. This work thus introduces tuned dichalcogenides as a platform strategy for enzyme-specific drug release from cell culture to in vivo, and brings the new chemical space of chalcogen redox into the med chem toolchest for bioreductive prodrugs.
38* L Zeisel*, M Maier, O Thorn-Seshold*;
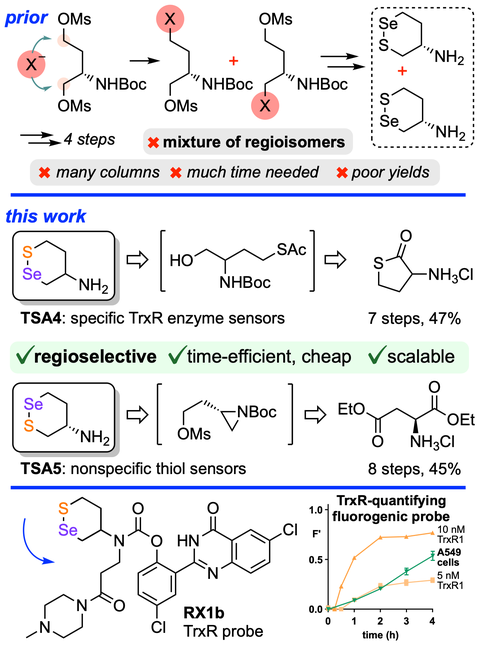
Regioselective, efficient and scalable syntheses of 1,2-thiaselenanes.
Synthesis 2023, 1385-1393 (jxfb) (pp, PDF )
1,2-thiaselenane-amines are valuable redox-active motifs for chemical biology, but their use is hindered by tedious synthesis. Here we develop their first regioselective syntheses, leveraging an aziridine intermediate and a kinetically controlled S-acylation for the regioselective chalcogen installations. Short, fast sequences with just 1-2 chromatographic steps cheaply deliver these motifs on scale for high-throughput inhibitor screening, and provide robust methods for other dichalcogenides.
37 F Coelho, S Saidjalolov, D Moreau, O Thorn-Seshold, S Matile*;
Inhibition of Cell Motility by Cell-Penetrating Dynamic Covalent Cascade Exchangers: Integrins Participate in Thiol-Mediated Uptake.
JACS Au 2023, 1010-1016 (j5sn)
Thiol-mediated uptake: which proteins are behind it? Until now, only the transferrin receptor TfR was known, but it seemed at best a minor player in TMU.
On a hunch, we tested if the redox-regulatable poly(Cys) integrin proteins offer the extracellular polyCys, flexibility, and self-clustering to be major TMU tracks. And they are! We reveal that the TMU of thiol/disulfide cascade exchanger motifs (CAXs) known since a decade is greatly abolished just by knocking out the β1 integrin - an astonishing home run for a hypothesis-driven shot in the dark. We also show that these CAXs block integrin-dependent cell migration with potency correlating to their TMU activity. This builds the first links between reversible thiol-reactivity as a principle for nature's macromolecules to enrich interactions at the cell surface, and how small molecule chemistry can harness or subvert it in subtle and useful ways.
36 M Wranik*, M Kepa*, et al, K Krauskopf, L Gao, O Thorn-Seshold, C Bostedt, C Bacellar, M Steinmetz, C Milne, J Standfuss*;
A multi-reservoir extruder for time-resolved protein crystallography and compound screening at X-ray free electron lasers.
Nature Communications 2023, 7956 (k68p)
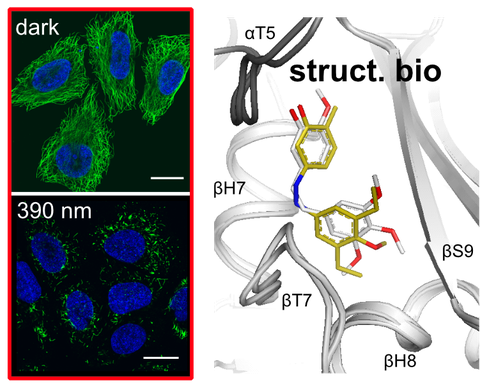
Serial crystallography at X-ray free-electron lasers (XFELs) is a room-temperature technique that allows determining time-resolved structures as ligands and proteins reorganise during photopharmaceutical switching, as well as detecting low occupancy ligands: both of which are inaccessible in standard cryo-crystallography.
Here, an extruder for automated XFEL delivery was developed and applied to identify low occupancy (≤30%) photoswitchable tubulin ligands whose binding mode (non/covalent) had been unclear. The results can now be exploited for rational structure-based design of photoswitchable antimitotic reagents.
35 C Velasco, R Santarella-Mellwig, M Schorb, L Gao, O Thorn-Seshold, A Llobet;
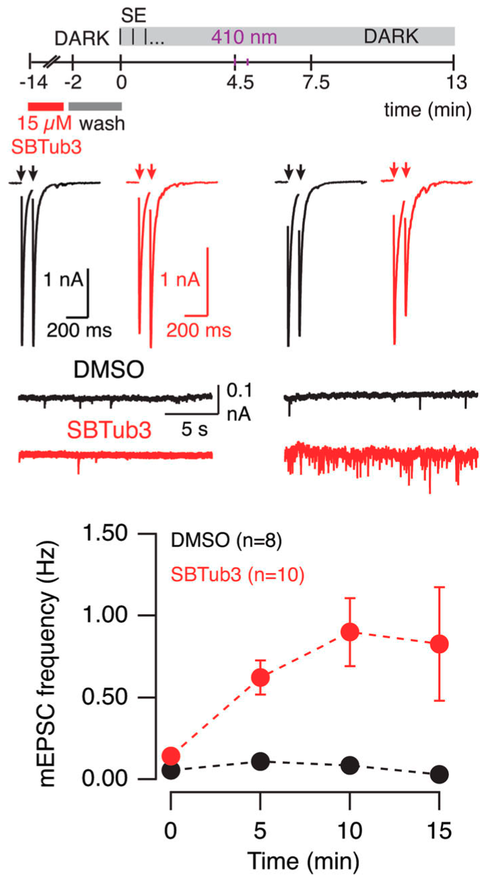
Microtubule depolymerization contributes to spontaneous neurotransmitter release.
Communications Biology 2023, 488 (kc77)
Microtubules underlie many neuronal functions involving the transport of organelles, however, their relationship to neurotransmitter release is still unresolved. Here, we show that microtubules present in the presynaptic compartment of cholinergic autaptic synapses are dynamic, and that the balance between microtubule growth and shrinkage affects neurotransmission. For this we induced synchronous microtubule depolymerization by photoactivation of SBTub3, which increased spontaneous neurotransmitter release (similar to effects of the motor depolymeriser Kif18A and the scaffolding depolymeriser stathmin-1). These results support that intact microtubules restrict spontaneous neurotransmitter release as well as promote the replenishment of the readily releasable pool of synaptic vesicles.
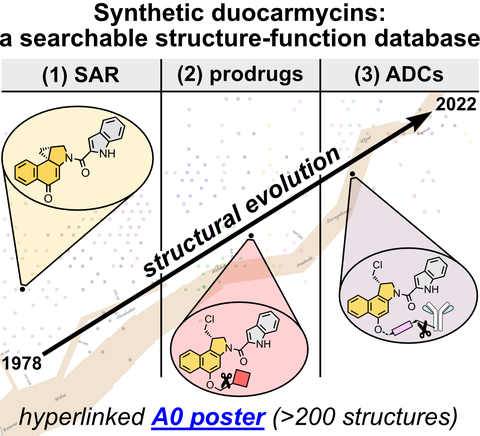
34* J Felber*, O Thorn-Seshold*;
40 Years of Duocarmycins: A Graphical Structure/Function Review of Their Chemical Evolution, from SAR to Prodrugs and ADCs.
JACS Au 2022, 2636–2644 (jmj2)
ACS Editors' Choice article; correction re. FDA status
A0 Poster: the Top 200 Duocarmycins
Duocarmycins are picomolar cytotoxins with fascinating, cyclisation-based bioactivity. We present an A0-size Poster "The Top 200 Duocarmycins", colour-coded by functional motifs, to narrate a structure-and-chronology overview of 600 research reports. The structures digitally hyperlink to their paper DOIs; or the vectorial poster can be printed to hang in hallways, for an accessible overview even without text. We concisely analyse their SAR, prodrugs, antibody-drug conjugates, and clinical trials, showing the duocarmycins as a platform with unique opportunities for rationally designed prodrugs and therapeutics.
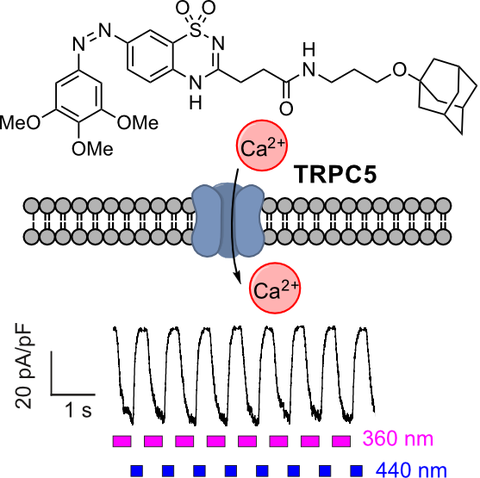
33* M Müller, K Niemeyer, N Urban, N Ojha, F Zufall, T Leinders-Zufall, M Schaefer, O Thorn-Seshold*;
BTDAzo - a photoswitchable TRPC5 channel activator.
Angewandte Chemie 2022, e202201565 (h2wb)
The TRPC5 calcium-permeable cation channel has distinct tissue-specific roles, from synaptic function to hormone regulation. Here we develop the first photoswitchable TRPC5-activating reagent, BTDAzo, which can photocontrol TRPC5-based neuronal calcium responses from cell culture to mouse brain slices, for high-precision research in TRP channel biology. (BTDAzos are also the first azo-benzothiadiazines, an accessible and conveniently derivatised azoheteroarene with excellent two-colour photoswitching).
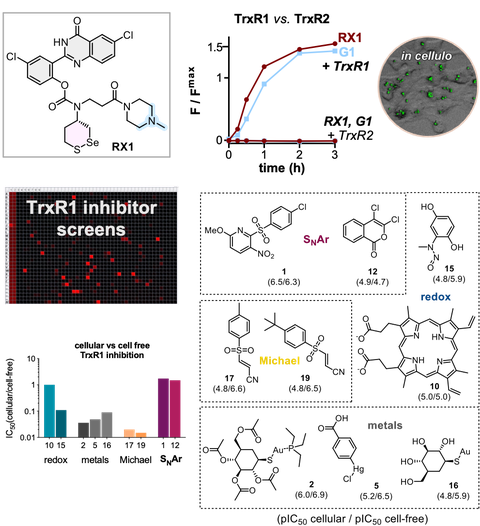
32* L Zeisel, J Felber, K Scholzen, L Poczka, D Cheff, M Maier, Q Cheng, M Shen, M Hall, E Arnér, J Thorn-Seshold, O Thorn-Seshold*;
Selective cellular probes for mammalian thioredoxin reductase TrxR1: rational design of RX1, a modular 1,2-thiaselenane redox probe.
Chem 2022, 1493-1517 (hq87) (preprint with Supp Info)
The answer is Selenium! The question: how to make small molecule probes that are selective cellular reporters of the unique selenol/thiol redox enzyme Thioredoxin Reductase (TrxR), an upstream driver in metabolism and disease?
We regiospecifically place a "dual-duty" selenium, to both (1) recruit the target selenol of TrxR, yet also (2) kinetically block monothiol reduction by futile cycling. We use the flipped regioisomer as a design control, highlighting the role of 5- vs 6-exo-trig cyclisation kinetics in avoiding reduction by non-target monothiols (with million-fold higher cellular concentrations than our target TrxR). By adding regioisomeric reactivity control to the dichalcogenide topology and geometry control we previously established, this paper delivers RX1: the first cellularly-selective bioreduction probe for mTrxR (cf. paper 55).
Lukas' Potential Energy highlight: 10.1016/j.chempr.2022.04.014 (gp4s84)
Highlights Hondal, Redox Biology 2022; Johansen-Leete & Payne, Chem 2022
31* L Gao, J Meiring, A Varady, I Ruider, C Heise, M Wranik, C Velasco, J Taylor, B Terni, J Standfuss, C Cabernard, A Llobet, M Steinmetz, A Bausch, M Distel, J Thorn-Seshold, A Akhmanova, O Thorn-Seshold*;
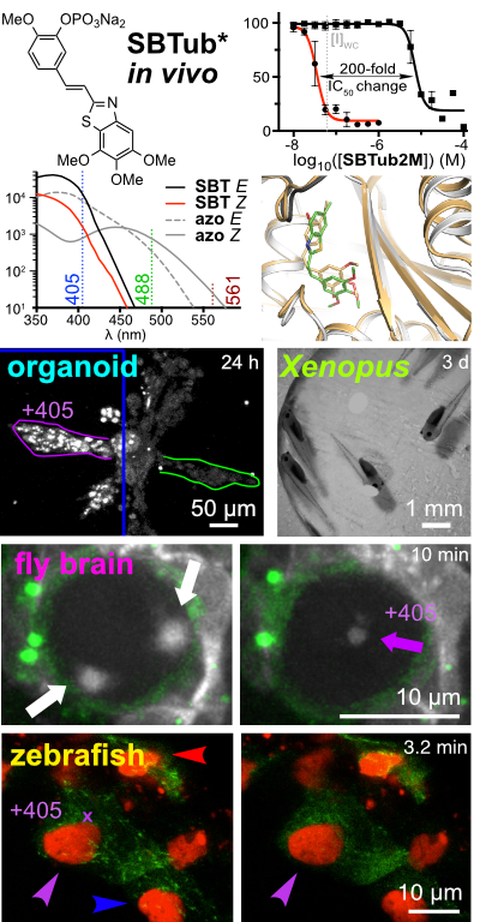
In vivo photocontrol of microtubule dynamics and integrity, migration and mitosis, by the potent GFP-imaging-compatible photoswitchable reagents SBTubA4P and SBTub2M.
Journal of the American Chemical Society 2022, 5614-5628 (hk66)
Photopharmaceuticals in 2D cell culture can deliver target-selective potency with spatiotemporal specificity, through local illumination of globally-treated samples. But in 3D and in vivo, this has essentially not been reproduced.
Here, we optimise a novel photoswitch for microtubule inhibition in vivo, ensuring it stays fully compatible with multiplexed fluorescent protein imaging. Its mechanism of action is reproducible from cell culture through to 3D systems (organoids, whole brain explant) through to classic animal models (zebrafish, clawed frog): systemic SBTub2M or SBTubA4P treatment plus spatiotemporally-localised illuminations photocontrol microtubule dynamics, MT network architecture, cell migration and cell division with cellular precision and second-level resolution in vivo. These in vivo-capable reagents can rewire how we aim for high-precision cytoskeleton research in cargo transport, cell motility, cell division and development. Their design will also be extended to a range of other protein targets: and general in vivo photopharmacology comes one step closer to reality!
30* J Felber, L Poczka, K Scholzen, L Zeisel, M Maier, S Busker, U Theisen, C Brandstädter, K Becker, E Arnér, J Thorn-Seshold, O Thorn-Seshold*;
Cyclic 5-membered disulfides are not selective substrates of thioredoxin reductase, but are opened nonspecifically by thiols.
Nature Communications 2022, 1754 (hn4h)
Cyclic five-membered disulfides (S50 dithiolanes) are kinetically labile motifs, that are widely used as "TRFS" redox probes for thioredoxin reductase (TrxR) e.g. in inhibitor screening and disease analysis.
Here we show that the past decade's papers interpreting them as TrxR reporters may need retraction. Dithiolane probes are nonspecifically reduced by a range of thiols and proteins, are barely affected by TrxR inhibition or knockout, and even switch on spontaneously by polymerisation. We show why misinterpretations have arisen, and we present a robust approach on which to build a future for redox research.
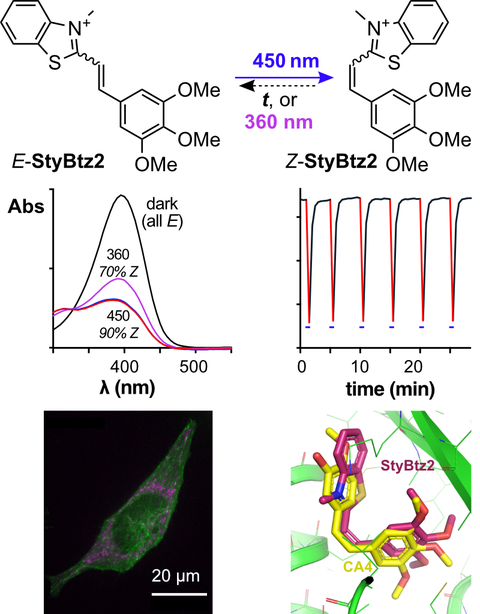
29* L Gao, Y Kraus, A Stegner, T Wein, C Heise, L von Brunn, E Fajardo-Ruiz, J Thorn-Seshold, O Thorn-Seshold*;
Self-reporting styrylthiazolium photopharmaceuticals: mitochondrial localisation as well as SAR drive biological activity.
Org Biomol Chem 2022, 7787-7794 (jjtb) (preprint )
Novel photoswitches are needed to drive advances in photopharmacology.
We present the first cellular trials of StyTz and StyBtz: E/Z-photoswitches that are near isosteres to azobenzenes, but have red-shifted π→π* bands, self-reporting fluorescence, Z→E relaxation on biological timescales, and decent solubility (charge). We tested StyTz and StyBtz designed for microtubule inhibition. Although they were light-specifically active, our studies excluded microtubules as a target. Using its self-reporting fluorescence, we assigned their light-dependent activity instead to mitochondrial photodisruption. StyTz/StyBtz may prove interesting photopharmaceutical scaffolds for mitochondrial targets, albeit not for cytosolic ones.
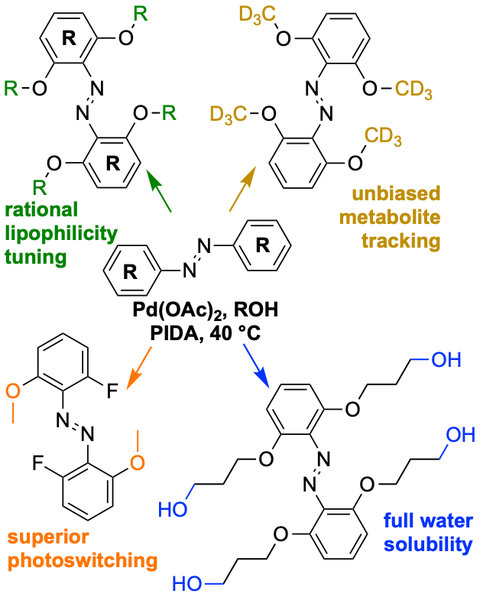
28* A Müller-Deku, O Thorn-Seshold*;
Exhaustive catalytic ortho-alkoxylation of azobenzenes: flexible access to functionally diverse yellow-light-responsive photoswitches.
Journal of Organic Chemistry 2022, 16526–16531 (js5z) (preprint )
Per-ortho-alkoxylated azobenzenes have attractive photoswitching and functional potential, but were a nightmare to synthesise systematically.
Here we developed the first method for per-ortho-alkoxylation of azobenzene photoswitches. This gives better E→Z and Z→E photoisomerisations and red-shifts photoresponses by >100 nm; but it also enables late-stage lipophilicity tuning, isotopic tracer preparation, or water solubility installation; and the M2F2 photoswitches we now access can also outperform 'gold standard' tetrafluoro- or tetrachloro- systems. Both the scaffolds and the method may thus broadly impact photopharmacology.
27* O Thorn-Seshold*;
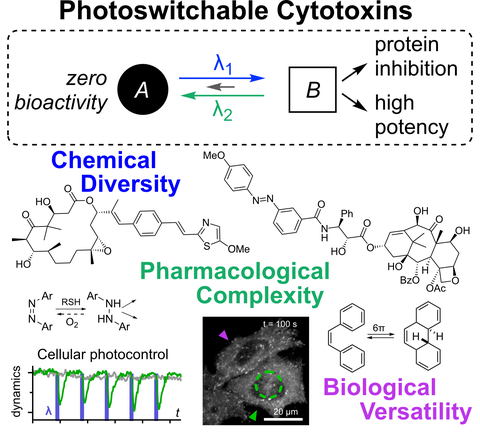
Chapter 36: Photoswitchable Cytotoxins (pp 873-919)
in Molecular Photoswitches, 2022 [Z Pianowski, Ed.; Wiley] (h2wc)
Cytotoxins are drugs whose targets are so essential to biology that inhibiting them causes cell death. Photoswitchable analogues of these cytotoxins promise valuable applications, leveraging high-precision optical control for unique modulation studies of these essential biological targets. However, they face stricter challenges in design and validation than other classes of photopharmaceuticals. Developments in the last decade have targeted the cytoskeleton, structural and genomic integrity, and signaling; and principles for the creation and use of these reagents have been clarified. The stage is set for further applications of photoswitchable cytotoxins in the life sciences.
26* O Thorn-Seshold*, J Meiring;
Chapter 26: Photocontrolling Microtubule Dynamics with Photoswitchable Chemical Reagents (pp 403-430)
in Microtubules, 2022 [H. Inaba, Ed.; Springer] (hsb2) (preprint)
A practical quick-start guide for biologists to use photoswitchable chemical reagents in cells. Optically controlling microtubules in vivo has gone from dream to reality in less than a decade. A range of photoswitchable chemical MT inhibitors from our group now allow sub-second and micron-scale spatiotemporal resolution of inhibition over MT dynamics and MT-dependent processes. However, there were still no user guides for biologists to establish photopharmaceutical assays; and the principles for high-quality assay design and validation have not been codified even in the chemistry community. We present:
(i) the basics of photoswitching small molecules;
(ii) their special performance, requirements, and limitations;
(iii) assay validation and benchmarking workflows including troubleshooting;
(iv) the major photoswitchable MT inhibitors available;
(v) workflows to establish cellular assays optically controlling MT dynamics with temporal reversibility and spatial specificity down to a single selected cell.
These methods are generally translatable to establishing and benchmarking assays with photopharmaceuticals for any protein target; and should help chemists to develop photoswitchable reagents more robustly, as well as equipping biologists to apply them further to 3D culture and in vivo.
25. J Rushworth, et al., O Thorn-Seshold, M Fuchter*;
[5]-Helistatins: Tubulin binding helicenes with antimitotic activity.
JACS Au 2022, 2561–2570 (jhgf)
Helicenes are scaffolds with high synthetic and technological interest, that have not yet reached applications as cellular bioactives. We developed a first-in-class antimitotic helicene, helistatin, as a mimic of colchicine. Helistatin blocks MT polymerisation cell-free and in live cells. This demonstrates the feasibility of using helicenes as bioactive scaffolds, with potential as near-isosteres of biaryls or cis-stilbenes.
24. M Scheck, et al., G Barba-Spaeth, O Thorn-Seshold, A Krug, S Endres, S Rothenfusser*, J Thorn-Seshold*;
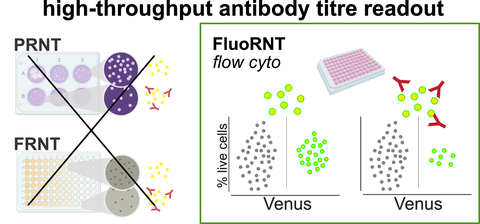
FluoRNT: A robust, efficient assay for the detection of neutralising antibodies against yellow fever virus 17D.
PLoS One 2022, 17, e0262149 (hgjw)
There is an urgent need for high-throughput, scalable, rapid, cheap and robust methods to quantify patient protective antibody titres against viral infection. We develop FluoRNT, a flow cytometry method using a fluorescent viral variant of yellow fever, that is 10-100-fold more efficient than current methods, fully automatisable, and minimises time of contact with patient samples. The assay system ensures significantly greater data precision and reproducibility than current methods, enabling sensitive quantitative studies.
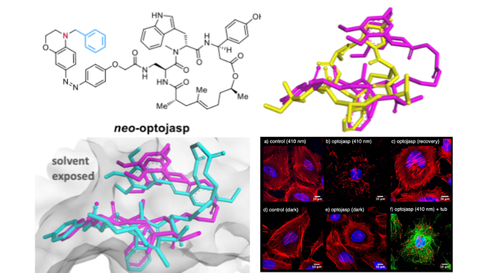
23. F Küllmer, N Vepřek, et al., O Thorn-Seshold, H-D Arndt, D Trauner;
Next Generation Opto-Jasplakinolides Enable Local Remodeling of Actin Networks.
Angewandte Chemie 2022, e202210220 (jbcz) (preprint )
We introduce 2nd generation photoswitchable actin inhibitors, neoOptoJasps, based on insights from CryoEM and SAR studies. They can be photoactivated with blue light, then relax spontaneously and rapidly to their less active isomer. They enable reversible control of F-actin dynamics and migration in live cells; and sub-cellular photoactivation in peripheral regions locally influences cytoskeleton stability and dynamics. These tools will empower spatiotemporally precise studies of the actin cytoskeleton.
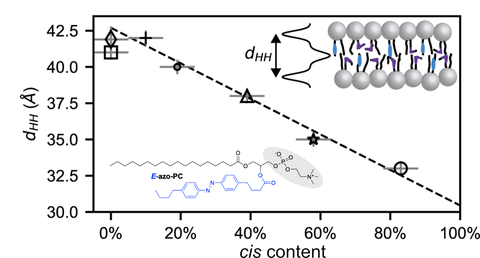
22. M Ober, A Müller-Deku, et al., O Thorn-Seshold, B Nickel;
SAXS measurements of azobenzene lipid vesicles reveal buffer-dependent photoswitching and quantitative Z→E isomerisation by X-rays.
Nanophotonics 2022, 2361-2368 (h2v6)
We study the switching of photoresponsive lipid membranes with small-angle X-ray scattering, showing that soft X-rays can quantitatively isomerise photolipid membranes to the all-trans state: both enabling more powerful X-ray-based membrane control and underlining the role of high energy X-rays for observation-only soft matter experiments.
21* L Gao, J Meiring, C Heise, A Rai, A Müller-Deku, A Akhmanova, J Thorn-Seshold, O Thorn-Seshold*;
Photoswitchable epothilone-based microtubule stabilisers allow GFP-imaging-compatible, optical control over the microtubule cytoskeleton.
Angewandte Chemie 2022 VIP paper, e202114614 (g94z)
Epothilone and Taxol, C=C photoswitches new and old: here, we bring the GFP-orthogonal azobenzene isostere styrylthiazole (ST) into photopharmacology. The nanomolar-potent STEpo reagents are photoswitchably microtubule-stabilising reagents with micron-precise targeting and intriguing potential towards in vivo applications.
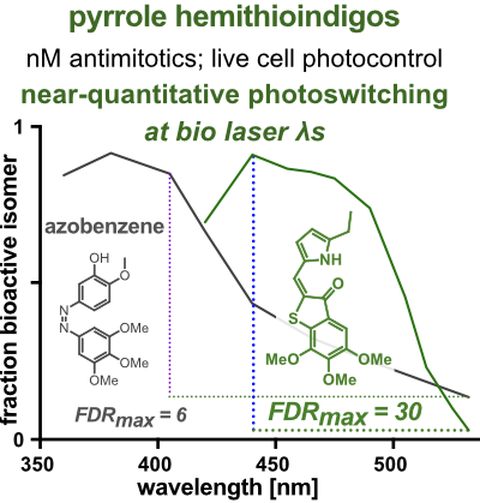
20* A Sailer, J Meiring, C Heise, L Pettersson, A Akhmanova, J Thorn-Seshold, O Thorn-Seshold*;
Pyrrole hemithioindigo antimitotics with near-quantitative bidirectional photoswitching photocontrol cellular microtubule dynamics with single-cell precision.
Angewandte Chemie 2021, 23695-23704 (gs63) , SynFacts
Most photopharmaceuticals suffer background activity, as their photoswitching is incomplete. Our PHTubs are near-quantitatively-switchable reagents that deliver cell-precise real-time photocontrol over microtubule dynamics, cell cycle, and cell death, for high-precision cytoskeleton research. This is the first use of hemithioindigos for high-resolution control in live cell assays, and shows PHTs may be excellent scaffolds for a range of other biological targets.
19* J Felber, L Zeisel, L Poczka, K Scholzen, S Busker, M Maier, U Theisen, C Brandstädter, K Becker, E Arnér, J Thorn-Seshold, O Thorn-Seshold*;
Selective, modular probes for thioredoxins enabled by rational tuning of a unique disulfide structure motif.
Journal of the American Chemical Society 2021, 143, 8791–8803 (gkbxdd)
Cyclic disulfides have a lot of potential for redox biology! Here we rationally design unique S6x disulfides, invoking a combination of considerations for topology, thermodynamics, and kinetics. These give the only disulfide-based probes that can resist the cellular monothiol background; and they proved to be selectively triggered by the central redox effector protein, thioredoxin (Trx). The methodology in this paper forms the basis for our longterm project on redox biology.
18* L Gao, J Meiring, Y Kraus, M Wranik, T Weinert, S Pritzl, R Bingham, E Ntouliou, K Jansen, N Olieric, J Standfüss, L Kapitein, T Lohmüller, J Ahlfeld, A Akhmanova, M Steinmetz, O Thorn-Seshold*;
A robust, GFP-orthogonal photoswitchable inhibitor scaffold extends optical control over the microtubule cytoskeleton.
Cell Chemical Biology 2021, 228-241 (f5qh); SynFacts (preprint )
It's no use photoswitching if you can't see what you're doing, or if the switch falls apart while you use it. These heterostilbene SBTubs were developed to leave GFP/YFP imaging channels free, and also resist in vivo metabolism. They allow exquisitely orthogonal photocontrol of cellular microtubule dynamics with minimal crosstalk/bleaching.
17* O Thorn-Seshold*, J Felber, J Thorn-Seshold, L Zeisel;
Disulfide prodrug compounds.
Patent 2021, WO 2022/200347 (EP21163944.8)
A modular probe & prodrug system using disulfide redox chemistry.
16* O Thorn-Seshold*, L Zeisel, J Felber;
Dichalcogenide Prodrugs.
Patent 2021, WO 2022/214598 (EP21167187.0)
A modular probe & prodrug system using dichalcogenide redox chemistry.
15* A Müller-Deku, J Meiring, K Loy, Y Kraus, C Heise, R Bingham, K Jansen, X Qu, F Bartolini, L Kapitein, A Akhmanova, J Ahlfeld, D Trauner, O Thorn-Seshold*;
Photoswitchable paclitaxel-based microtubule stabilisers allow optical control over the microtubule cytoskeleton.
Nature Communications 2020, 4640 (gnsd)
These AzTax reagents (azobenzene-extended taxols) are the first photoswitchable microtubule stabilisers. They enable cellularly- and subcellularly-specific photocontrol of MT polymerisation dynamics, incl. in primary neurons.
14* Y Kraus#, C Glas#, B Melzer, L Gao, C Heise, M Preuße, J Ahlfeld, F Bracher, O Thorn-Seshold*;
Isoquinoline-based biaryls as a robust scaffold for microtubule inhibitors.
European Journal of Medicinal Chemistry 2020, 111865 (h2wd) (preprint )
Colchicinoids have two aryl blades separated by a bridge that is often metabolically or photochemically labile. These IQTub biaryls are highly robust; easy to make and diversify; and are potent druglike cellular binders.
13* A Sailer, F Ermer, Y Kraus, R Bingham, F Lutter, J Ahlfeld, O Thorn-Seshold*;
Potent hemithioindigo-based antimitotics photocontrol the microtubule cytoskeleton in cellulo.
Beilstein Journal of Organic Chemistry 2020, 125 (hkx8)
Improving on our first generation photoswitchable microtubule inhibitor HTIs, these HITubs are mid-nanomolar cell-active photoswitchable tubulin binders with all-visible-light switching, illustrating the potential of the HTI scaffold for cell biology.
12. U Theisen, A Ernst, R Heyne, T Ring, O Thorn-Seshold, R Köster;
Microtubules and motor proteins support zebrafish neuronal migration by directing cargo.
Journal of Cell Biology 2020, e201908040 (h2v7)
Not only motile cell types but also neurons require dynamic MTs during motion. Spatiotemporally precise inhibition of their MTs with PST-1 links MT-based protein relocalisation to migration speed and directionality.
11. M Borowiak, F Küllmer, F Gegenfurtner, S Peil, V Nasufovic, S Zahler, O Thorn-Seshold, D Trauner, H-D Arndt;
Optical manipulation of F-actin with photoswitchable small molecules.
Journal of the American Chemical Society 2020, 9240 (gzht)
These OptoJasps (azobenzene-extended jasplakinolides) are the first photoswitchable inhibitors for actin dynamics. They enable cellularly-targeted modulation of cell motility and other actin-dependent processes.
10. A Kopf, J Renkawitz, R Hauschild, I Girkontaite, K Tedford, J Merrin, O Thorn-Seshold, D Trauner, H Häcker, K-D Fischer, E Kiermaier, M Sixt;
Microtubules control cellular shape and coherence in amoeboid migrating cells.
Journal of Cell Biology 2020, 219, e201907154 (ggzn8n) (preprint )
Using PSTs to spatiotemporally precisely inhibit MT dynamics in highly motile cells revealed the roles of MTs in maintaining cell shape and coordinating retractions with actomyosin.
9. M Bischof, S Olthoff, C Glas, O Thorn-Seshold, M Schaefer, K Hill;
TRPV3 endogenously expressed in murine colonic epithelial cells is inhibited by the novel TRPV3 blocker 26E01.
Cell Calcium 2020, 92, 102320 (h2v8)
26E01 was identified as a nontoxic blocker of TRPV3 currents in overexpression and native-expression cellular systems, that does not affect TRPV1, TRPV2, and TRPV4 channels.
8* O Thorn-Seshold*;
Comment on “Photo-Controlled Reversible Microtubule Assembly”.
Angewandte Chemie 2020, 59, 7652-7654 (h2wf)
Metabolically labile inhibitor-photoswitch attachment strategies and PAINS-like aggregation modulation can confound reagent design. This critique to Liu et al., ACIE 2018 highlights the danger of neglecting controls and SAR. Despite their own followup showing that non-inhibitors give identical effects as claimed inhibitors (Liu et al., ACIE 2019), neither corrections nor retractions were issued, and their citation net continues (author response here).
7* A Sailer, F Ermer, Y Kraus, F Lutter, C Donau, M Bremerich, J Ahlfeld, O Thorn-Seshold*;
Hemithioindigos for cellular photopharmacology: desymmetrised molecular switch scaffolds enabling design control over the isomer-dependency of potent antimitotic bioactivity.
ChemBioChem 2019, 1305-1314 (hb5v)
The understudied hemithioindigos have more attractive photoswitching than standard azobenzenes. These HOTubs are the first HTI-pharmacophore reagents to succeed in cell biology, and enable desymmetrised designs.
6* O Thorn-Seshold*, F. Bracher, B. Melzer;
Isoquinoline biaryl compounds.
Patent 2018, EP18207030
IQTubs are diversifiable, metabolically robust isosteres for cis-stilbenoid bioactives.
5. A Singh, T Saha, I Begemann, A Ricker, H Nüsse, O Thorn-Seshold, J Klingauf, M Galic, M Matis;
Polarized microtubule dynamics directs cell mechanics and coordinates forces during epithelial morphogenesis.
Nature Cell Biology 2018, 20, 1126 (gfcc9p)
Spatiotemporally resolved, reversible PST photoswitching elucidates the need for dynamic MTs in force generation to orient drosophila tissue development.
4. J Zenker, MD White, RM Templin, RG Parton, O Thorn-Seshold, S Bissiere, N Plachta;
A microtubule-organizing center directing intracellular transport in the early mouse embryo.
Science 2017, 357, 925 (gbwmgk)
Spatiotemporally resolved, reversible PST photoswitching elucidates the location and origin of the non-centrosomal MT-organising system in the early stages of mammalian development.
3. K Eguchi, Z Taoufiq, O Thorn-Seshold, et al., T Takahashi;
Wild-type monomeric α-synuclein can impair vesicle endocytosis and synaptic fidelity via tubulin polymerization at the calyx of Held.
The Journal of Neuroscience 2017, 37, 6043 (gbjstw)
Temporally reversible MT inhibition applied using PST photoswitching contributes to understanding vesicle trafficking dynamics in neurons.
2* M Borowiak, et al., D Trauner*, O Thorn-Seshold*;
Photoswitchable Inhibitors of Microtubule Dynamics Optically Control Mitosis and Cell Death.
Cell 2015, 162, 403-411 (f7kcps)
Reversible photoswitching of PSTs allows bidirectionally reversible optical modulation of MT dynamics in live cells, and modifies cell division sequences during embryonic development.
1* O Thorn-Seshold*, M Borowiak, D Trauner, J Hasserodt;
Azoaryls as Reversibly Modulatable Tubulin Inhibitors.
Patent 2014, WO2015166295
Reversibly photoswitchable isosteres of stilbenoid colchicine-mimic drugs ("PSTs") for in vitro and in vivo control of microtubule dynamics.