Biological Adhesion and Motility
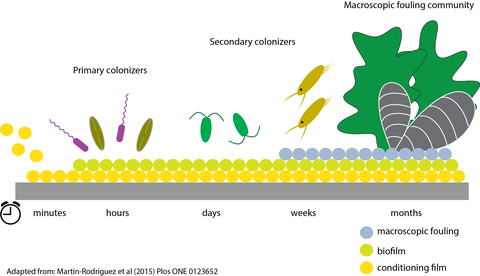
Marine Biofilm
Benthic, pennate diatoms are well known for their adhesion strength to natural and man-made surfaces. Much of the research on diatom adhesion has been driven by the need to develop anti-fouling surfaces that cannot be colonized by diatoms. On the other hand, diatom adhesives are highly attractive as a versatile bonding agent that adheres strongly to both hydrophobic and hydrophilic surfaces under water.
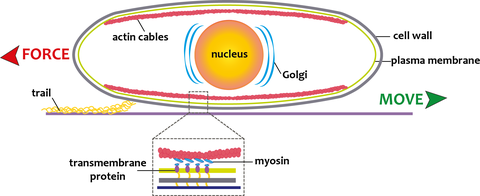
Diatom Adhesion/Motility model
Diatom adhesion is accomplished through an adhesion/motility complex that includes an intracellular bundle of actin fibers and on the extracellular side mucilage strands that project through a slit in the cell wall (raphe). The mucilage strands attach the cell to the surface, providing the traction for gliding movement of the cell through an as yet unidentified connection of the mucilage strands to the actin bundle. As diatoms glide across a substratum they leave behind a trail of the adhesive mucilage that can be easily visualized using the organic dyes Stains All and Alcian Blue, or fluorescently labeled lectins (i.e., carbohydrate binding proteins) and monoclonal antibodies that were raised against extracellular polymeric substances (EPS) from diatoms. These data suggest that diatom trails contain acidic glycans that are part of polysaccharide molecules and/or glycoproteins.
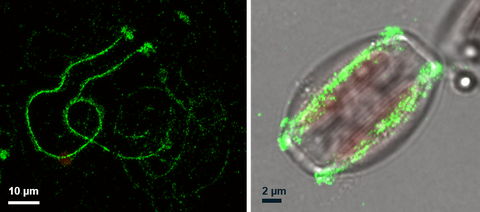
Immunolocalization of the Amphora coffeaeformis trail protein Ac629
We have recently developed a novel method to isolate the adhesive trails and revealed that they contain about 70% carbohydrate and 30% protein. Proteomics analysis of the purified adhesive material has identifed a number of novel proteins that do not share high similarity with other known proteins but do contain some domains that are present in adhesive proteins from other organisms (e.g. PTS-rich, von Wilebrand domains, Choice of anchor A domain, GDPH motif).
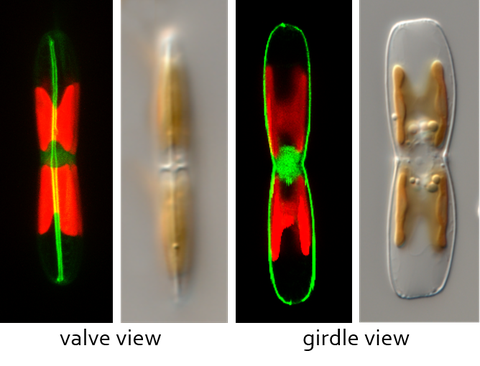
LifeAct_GFP labelling of the actin cytoskeleton in Craspedostauros australis
The power for diatom motility is hypothesized to be generated by the motor protein myosin which connects the intracellular actin cytoskeleton, via transmembrane components, to the extracellular adhesive proteins that provide the traction for cell movement. The force generated by this so called 'adhesion/motility complex' parallel to the actin filaments results in movement of the cell in the opposite direction. However, the identity of the myosin motor proteins involved in this process remains unknown. Currently we are developing the raphid pennate diatom Craspedostauros australis as a model system for the study of diatom motility. To this end we have performed transcriptomic and genomic sequencing and established a method for its genetic transformation.
