Feb 05, 2025
“Tubulin Code” Responsible for Avoidance of Head-on Collisions in Cilia
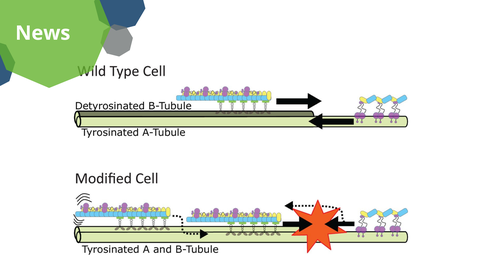
A visualization of the proposed model for the mechanism of intraflagellar transport along microtubule doublets in cilia. In wild type cells, the forward trains are loaded and are kept on the detyrosinated B-tubule, while the backward trains are loaded and kept on the tyrosinated A-tubule. In modified cells, the trains are not kept on distinct tubules, causing collisions and “traffic jams”.
By developing a sophisticated in vitro system that combines advanced imaging techniques and CRISPR genome editing, an international team of researchers from the B CUBE – Center for Molecular Bioengineering and Human Technopole (Italy) explained how collisions between intraflagellar trains moving in opposite directions inside the cilium are avoided. They showed that posttranslational modifications of tubulin are responsible for the regulation of bidirectional cargo train movements in cilia. The research was funded by the ERC and the DFG Cluster of Excellence Physics of Life. The results are published in Nature Communications.
Cilia are hair-like organelles found on nearly all eukaryotic cells. They play crucial roles in cell signaling, movement, and fluid transport. Proper assembly and function of cilia rely on a sophisticated bidirectional transport system called intraflagellar transport (IFT). Disruptions in intraflagellar transport are linked to a variety of disorders, collectively known as ciliopathies, which include respiratory diseases, infertility, obesity, diabetes, cardiovascular disease, sensory impairments, and neurological disorders. Researchers study cilia to understand which mechanisms control cargo transport to improve their understanding of such diseases.
Just like in a railway station, the intraflagellar transport system uses microtubule tracks to ensure the ordered movement of molecular cargos between the cell body and the tip of the cilium. But how is this complex traffic ordered and what mechanisms are in place to make sure that cargos transported in opposite directions do not collide?
Post-Translational Modification is Key
The groups of Stefan Diez, Professor for BioNano-Tools at the B CUBE – Center for Molecular Bioengineering of TU Dresden, and Gaia Pigino, Associate Head of the Structural Biology Research Centre at Human Technopole in Milan, investigated the role of tubulin post-translational modifications in regulating intraflagellar transport.
The researchers demonstrated that detyrosination, a modification enriched on specific microtubules within the cilium, is critical for the smooth transport operation. Using modified Chlamydomonas reinhardtii algae that lacked tubulin detyrosination, they observed frequent stops of intraflagellar transport trains and misrouting of cargos. These disruptions caused collisions among trains moving in opposite directions and hindered ciliary growth.
Further experiments using synthetic microtubules in vitro revealed that forward trains (which move toward the ciliary tip) prefer detyrosinated microtubules. In contrast, backward trains (which move back to the cell body) favor tyrosinated ones. This differential affinity helps segregate the two train types onto distinct microtubules, ensuring collision-free transport. In modified cells, this segregation was no longer there, causing traffic jams and functional impairment of the cilia.
Reconstituting the Transport Outside the Cell
This study is the first evidence linking tubulin detyrosination to the spatial organization of intraflagellar transport trains. Moreover, the study introduces a cutting-edge method to reconstitute train motility on synthetic microtubules, providing a controlled system to study these interactions at a molecular level.
Additionally, the approach to reconstitute intraflagellar transport in vitro offers a versatile platform for future research, because this method can be adapted to study other post-translational modifications and their effects on microtubule-associated processes.
“This study would not have been possible without the invaluable expertise of our partners,” says Prof. Diez. “Collaborating with the Pigino group - first at the Max Planck Institute of Molecular Cell Biology and Genetics in Dresden and now at the Human Technopole in Italy - has been a truly rewarding experience. This project seamlessly combined their expertise in molecular biology and electron microscopy with our strengths in in-vitro reconstitution and light microscopy."
By revealing how tubulin modifications direct transport logistics, the study addresses a long-standing question in the field of cilia biology. The results may have implications beyond basic science, as defects in ciliary transport are central to ciliopathies, such as polycystic kidney disease, primary ciliary dyskinesia, and retinal degeneration. The study's findings highlight how molecular fine-tuning within cilia is critical for their proper function, with broader implications for understanding diseases beyond ciliopathies, such as neurodegenerative disorders and cancer.
Original Publication:
Aditya Chhatre, Ludek Stepanek, Adrian Pascal Nievergelt, Gonzalo Alvarez Viar, Stefan Diez and Gaia Pigino: Tubulin tyrosination/detyrosination regulate the affinity and sorting of intraflagellar transport trains on axonemal microtubule doublets. Nature Communications (January 2025)
Link: https://doi.org/10.1038/s41467-025-56098-0
