01.12.2022
Mini-Netzhaut 2.0: Neues Modell zur Erforschung menschlicher Augenkrankheiten deckt bisher unbekannten Mechanismus des Sehverlusts auf
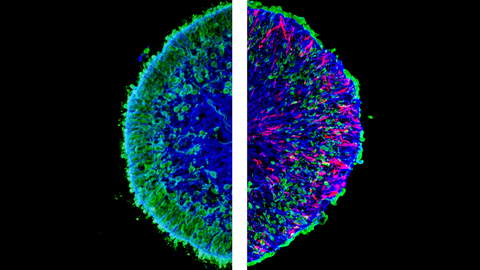
Mikroskopische Aufnahmen von im Labor gezüchteten Mini-Netzhäuten, so genannten menschlichen Netzhaut-Organoiden. Das linke Bild zeigt einen Schnitt eines gesunden (Kontroll-)Organoids. Das rechte Bild zeigt einen Organoidschnitt mit pathologischen Veränderungen. Auf der rechten Seite ist ein sichtbarer Verlust an grüner Farbe zu erkennen, der dem massiven Verlust von Photorezeptorneuronen entspricht, die grün markiert sind. Die rote Farbe zeigt die pathologischen Müller-Glia-Zellen.
Dresdner Forschende haben eine neue Generation menschlicher Mini-Netzhäute entwickelt und damit ein neues Modell für eine unheilbare menschliche Augenkrankheit geschaffen, sowie einen unbekannten Mechanismus des Sehverlusts entdeckt.
Wissenschaftler:innen aus Dresden haben ein Forschungsmodell entwickelt, das neue Möglichkeiten für die Untersuchung von Sehverlust eröffnet. Die im Labor gezüchteten Mini-Netzhäute sind die Ersten, die wichtige Merkmale der Makula aufweisen. Mit dem neuen System haben die Forschenden einen neuen Weg gefunden, komplexe krankhafte Veränderungen in der Netzhaut hervorzurufen und einen bisher unbekannten Mechanismus des Sehverlusts zu beschreiben. Die Mini- Netzhäute bieten ein potenzielles erstes Modell zur Untersuchung der altersbedingten Makuladegeneration (AMD) in einem menschlichem Netzhaut-Modell. Die Arbeit unter der Leitung von Prof. Mike O. Karl vom Zentrum für Regenerative Therapien Dresden (CRTD) der TU Dresden und dem Deutschen Zentrum für Neurodegenerative Erkrankungen (DZNE) wurde in der Zeitschrift Nature Communications veröffentlicht.
Das Geheimnis des hochauflösenden Sehens des Menschen liegt in einem kleinen Bereich im hinteren Teil unserer Augen, der so genannten Makula. Dieser winzige Teil unserer Netzhaut ist einzigartig für den Menschen. Er enthält eine große Anzahl von Zapfen-Photorezeptorzellen, die uns das hochauflösende Farbensehen ermöglichen. Der Verlust der Photorezeptoren ist das Schlüsselsymptom unheilbarer, zu Erblindendung führender Krankheiten wie der altersbedingten Makuladegeneration (AMD) und anderer erblicher Krankheiten. Sind die Photorezeptoren erst einmal verloren, können sie weder ersetzt werden noch nachwachsen. Das Sehvermögen geht allmählich verloren.
„Es wird geschätzt, dass jeder vierte Mensch über 60 Jahren an AMD leidet, mit derzeit etwa 67 Millionen Patient:innen in Europa", sagt Prof. Mike O. Karl, Forschungsgruppenleiter am Zentrum für Regenerative Therapien Dresden (CRTD) der TU Dresden und dem Deutschen Zentrum für Neurodegenerative Erkrankungen (DZNE). Trotzdem ist die AMD noch immer nicht gut genug verstanden, um eine wirksame Therapie zu entwickeln. Da die Makula nur beim Menschen vorkommt, ist die Untersuchung von Makulaerkrankungen in Tiermodellen nicht optimal. Der Mangel an anderen Forschungssystemen, die die menschliche Makula besser abbilden, ist die größte Herausforderung, die es zu bewältigen gilt.
„Zusammen mit meinem Team haben wir nun ein neues menschliches Netzhaut-Modell entwickelt, das mehrere Merkmale einer Schlüsselregion der menschlichen Makula reproduziert. Wir zeigen zum ersten Mal, dass ein solches Modell die Untersuchung von krankhaften Veränderungen ermöglicht, die bei Krankheiten wie AMD auftreten", fügt Prof. Karl hinzu.
Neue Generation von Mini-Netzhäuten
Organoide sind im Labor gezüchtete Miniorgane, die durch eine Technologie namens Reprogrammierung aus praktisch jeder menschlichen Zelle erzeugt werden können. Ein im Labor gezüchtetes Organoid enthält mehrere Zelltypen und reproduziert die charakteristischen Merkmale des Organs, das es nachahmt. Dadurch können sich die Forschenden auf die Zell-Zell-Interaktionen konzentrieren und komplexe Krankheitsmechanismen im menschlichen Umfeld abbilden.
Die neue Generation von Netzhaut-Organoiden, die von Prof. Karl und seiner Gruppe entwickelt wurde, ist reich an Zapfen-Photorezeptoren und reproduziert mehrere Schlüsselparameter einer Unterregion der Makula, der so genannten Parafovea, in der die AMD beginnt. „Zum ersten Mal haben wir ein Modell mit einigen Merkmalen einer entscheidenden Region der menschlichen Netzhaut. Wir zeigen, dass diese Organoide verwendet werden können, um komplexere Pathologien des Sehverlusts zu untersuchen, wie sie bei AMD beobachtet werden."
„Bisher entsprachen Netzhaut-Organoide meist den peripheren Teilen der menschlichen Netzhaut. Teile der Makula - der Schlüsselregion für das hochauflösende Sehen - wurden nicht abgebildet", erklärt Prof. Karl. „Im Vergleich zu anderen Teilen der Netzhaut hat die Makula viele spezielle Eigenschaften. Wir gehen davon aus, dass sich krankhafte Veränderungen dort anders entwickeln könnten."
Ein robustes neues System zur Förderung der AMD-Forschung
Die Forschung zeigt, dass Photorezeptoren nicht die einzige Art von Zellen sind, die bei Sehkraftverlust nicht mehr richtig funktionieren. „Die Nachbildung der Interaktion zwischen mehreren verschiedenen Zelltypen macht es schwierig, Sehkraftverlust zu untersuchen und - letztendlich - zu verstehen und zu heilen", erklärt Prof. Karl.
Die Forscher entdeckten, dass zwei Proteine, HBEGF und TNF (HT), komplexe pathologische Veränderungen in menschlichen Mini-Retinas hervorrufen. Die HT-Proteine wurden bereits früher bei neurodegenerativen Erkrankungen nachgewiesen, aber es war nicht bekannt, dass sie ausreichen, um eine Pathologie zu verursachen. „Wir haben einen fortschreitenden Verlust von Photorezeptorzellen beobachtet. Darüber hinaus sahen wir parallele Veränderungen bei anderen Zellen der Netzhaut, insbesondere bei Müller-Glia. Sie begannen sich zu teilen und bildeten narbenartige Läsionen, die die verlorenen Photorezeptoren ersetzten", sagt die Hauptautorin der Studie Dr. Manuela Völkner.
„All dies sind Merkmale der Netzhautdegeneration bei AMD und der Schäden, die im Spätstadium der meisten anderen Netzhauterkrankungen beobachtet werden. Während diese Prozesse bei Patientinnen und Patienten Jahre brauchen, um sich zu entwickeln, haben wir vergleichbare Veränderungen in Photorezeptoren und Gliazellen innerhalb eines einzigen Monats reproduziert", fügt Dr. Völkner hinzu. Das Team glaubt, dass diese Veränderung des Zeitrahmens für die Untersuchung von Krankheitsmechanismen von Vorteil ist. Dadurch können die Dynamik der krankhaften Veränderungen und die Ergebnisse möglicher Eingriffe schneller beobachtet werden.
Zum Sterben rausgeworfen – Sehverlust durch Degeneration der Photorezeptoren
Innerhalb von 10 Tagen nach der Stimulation mit HT-Faktoren war bereits die Hälfte aller Photorezeptoren in den Mini-Netzhäute verloren gegangen. Das Team erwartete, eine Menge toter Zellen in der Netzhaut zu finden. Sie konnten jedoch kaum welche finden. „Es sah aus, als ob sie verschwunden wären. Diese Beobachtung hat uns wirklich verblüfft", sagt Prof. Karl.
Das Team verfolgte die Zellen über einen längeren Zeitraum und setzte eine Reihe empfindlicher Techniken ein, um herauszufinden, dass die Photorezeptoren nicht wirklich in der Netzhaut absterben. Sie werden aus der Netzhaut verdrängt, bevor sie sterben. „Bislang hat noch niemand einen solchen Mechanismus bei neurodegenerativen Erkrankungen nachgewiesen", erklärt Prof. Karl.
„Wenn wir auf die Studien zurückblicken, in denen die Netzhäute von Patientinnen und Patienten analysiert wurden, können wir jetzt viele Veränderungen sehen, die auf diesen Mechanismus der Verdrängung der Photorezeptoren hindeuten. Dieser Prozess könnte daher möglicherweise bei der Alterung, der AMD und einigen erworbenen sowie vererbten Netzhauterkrankungen beteiligt sein", erklärt Prof. Karl. Es bleibt abzuwarten, ob die neue Beobachtung als potenzieller klinischer Biomarker und therapeutisches Ziel zur Verhinderung von Sehverlust genutzt werden kann.
Eine Brücke zwischen den Modellorganismen und dem Menschen
„Organoide bieten die einzigartige Möglichkeit, die Dynamik der Degeneration der menschlichen Netzhaut zu beobachten und zu verstehen", sagt Felix Wagner, Autor der Studie, welcher Videos vom Verlust menschlicher Photorezeptoren in Aktion mit dem Live-Mikroskop aufnimmt.
„Wir haben das erste menschliche Modell entwickelt, das die komplexen und fortschreitenden Merkmale neurodegenerativer Erkrankungen wie der AMD reproduzieren kann. Die Mini-Retinas bieten neue Möglichkeiten, um die Grundlagenforschung und die translationale Medizin zu erleichtern. Sie bieten einen neuen Ausgangspunkt für die Entwicklung von medikamenten-, gen- und zellbasierten Therapien und möglicherweise sogar ein vollständiges Modell der komplexen Makula in der Zukunft", fasst Prof. Karl zusammen.
Die Zusammenarbeit
Das Team betont, dass die Arbeit ohne Expert:innen aus Partnerinstitutionen und spezialisierte Forschungseinrichtungen nicht möglich gewesen wäre. Die langjährige Zusammenarbeit mit dem Team von Prof. Jörg Hackermüller an der Universität Leipzig und dem Helmholtz-Zentrum für Umweltforschung (UFZ) war entscheidend für die Studie.
Das CRTD und das DZNE in Dresden bieten die Möglichkeit, modernste Ressourcen zu nutzen. „Durch die Zusammenarbeit mit Fachkräften aus den Bereichen Stammzelltechnik, Durchflusszytometrie, Next-Generation-Sequencing, Elektronen- und Lichtmikroskopie sowie Bildanalyse konnten wir mit diesem Projekt große Sprünge nach vorne machen", sagt Prof. Karl.
Finanzierung
Die Forschung wurde von der Deutschen Forschungsgemeinschaft (DFG) im Rahmen des Schwerpunktprogramms SPP2127: Gen- und zellbasierte Therapien gegen neuroretinale Degeneration gefördert. Sie wurde auch durch das ERA-NET Neuron-Forschungskonsortium des Bundesministeriums für Bildung und Forschung (BMBF) ReDiMoAMD gefördert und vom CRTD der TU Dresden, DZNE Dresden und anderen Förderungen unterstützt.
Originalveröffentlichung
Manuela Völkner, Felix Wagner, Lisa Maria Steinheuer, Madalena Carido, Thomas Kurth, Ali Yazbeck, Jana Schor, Stephanie Wieneke, Lynn J. A. Ebner, Claudia Del Toro Runzer, David Taborsky, Katja Zoschke, Marlen Vogt, Sebastian Canzler, Andreas Hermann, Shahryar Khattak, Jörg Hackermüller & Mike O. Karl: HBEGF-TNF induce a complex outer retinal pathology with photoreceptor cell extrusion in human organoids.
Nature Communications (Oktober 2022)
Link: https://doi.org/10.1038/s41467-022-33848-y
Über das Zentrum für Regenerative Therapien Dresden (CRTD)
Am Zentrum für Regenerative Therapien Dresden (CRTD) der TU Dresden widmen sich Spitzenforscher und -forscherinnen aus mehr als 30 Ländern neuen Therapieansätzen. Sie entschlüsseln die Prinzipien der Zell- und Geweberegeneration und ergründen deren Nutzung für Diagnose, Behandlung und Heilung von Krankheiten. Das CRTD verknüpft Labor und Klinik, vernetzt Wissenschaft und Klinik, nutzt Fachwissen in Stammzellforschung, Entwicklungs- und Regenerationsbiologie, um letztlich die Heilung von Erkrankungen wie Alzheimer und Parkinson, hämatologischen Krankheiten wie Leukämie, Stoffwechselerkrankungen wie Diabetes sowie Augen- und Knochenerkrankungen zu erreichen.
Das CRTD wurde 2006 als Forschungszentrum der Deutschen Forschungsgemeinschaft (DFG) gegründet und bis 2018 als DFG-Forschungszentrum, sowie als Exzellenzcluster gefördert. Seit 2019 wird das CRTD mit Mitteln der TU Dresden und des Freistaates Sachsen finanziert.
Das CRTD ist eines von drei Instituten der zentralen wissenschaftlichen Einrichtung Center for Molecular and Cellular Bioengineering (CMCB) der TU Dresden.
Web: www.tu-dresden.de/cmcb/crtd
Web: www.tu-dresden.de/cmcb
Über das Deutsche Zentrum für Neurodegenerative Erkrankungen (DZNE)
Das DZNE ist ein von Bund und Ländern gefördertes Forschungsinstitut, das bundesweit zehn Standorte umfasst. Es widmet sich Erkrankungen des Gehirns und Nervensystems wie Alzheimer, Parkinson und ALS, die mit Demenz, Bewegungsstörungen und anderen schwerwiegenden Beeinträchtigungen der Gesundheit einhergehen. Bis heute gibt es keine Heilung für diese Erkrankungen, die eine enorme Belastung für unzählige Betroffene, ihre Familien und das Gesundheitssystem bedeuten. Ziel des DZNE ist es, neuartige Strategien der Vorsorge, Diagnose, Versorgung und Behandlung zu entwickeln und in die Praxis zu überführen. Dafür kooperiert das DZNE mit Universitäten, Universitätskliniken und anderen Institutionen im In- und Ausland. Das Institut ist Mitglied der Helmholtz-Gemeinschaft und zählt zu den Deutschen Zentren der Gesundheitsforschung.
Zusätzliche Materialien:
Website der Forschungsgruppe von Prof. Mike O. Karl: https://tud.de/cmcb/crtd/karl
Bildmaterial: https://tud.link/vwqq
Wissenschaftlicher Ansprechpartner:
Prof. Mike O. Karl
Center for Regenerative Therapies Dresden (CRTD)
Technische Universität Dresden
Fetscherstr. 105, 01307 Dresden
Tel: +49 (0) 351 210463-604
Email:
