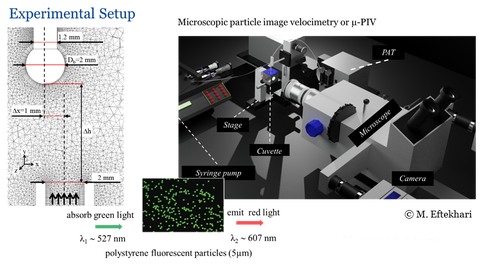
Interfacial properties of droplets and bubbles under flow conditions
Contact person: Prof. Dr. et Ing. habil. Kerstin Eckert, Dr.-Ing. Karin Schwarzenberger
Project staff: Dipl.-Ing. Milad Eftekhari
Motivation
Bubbles and drops are the basic elements of many industrial applications. The interfacial properties of these bubbles and drops define the efficiency of the process, from the stability of the foams and emulsions in cosmetics, pharmaceutics, and food to the heat and mass transfer in mineral processing, drug delivery, and liquid-liquid separation. In practice, bubbles and drops are under dynamic conditions; however, their behavior is mainly studied under quasi-static condition. Here, we investigate the behavior of droplets and bubbles under flow conditions, with the focus on asymmetric shear flow.
Methods
- Particle image/tracking velocimetry (PIV/PTV) for flow measurements.
- Profile analysis tensiometry (PAT) for interfacial tension/rheology measurements.
Results
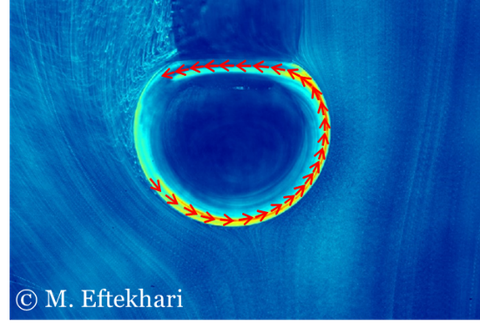
Interfacial flow on bubble surface visualized by tracking the PS particles.
- Asymmetric shear present inside a reactor can influence surfactant distribution at the interface.
- We visualize a continuous circulation at the interface under asymmetric shear flow.
- The interface remains mobile regardless of the surfactant concentration and the so-called stagnant cap, which drastically can decrease the rising velocity of bubbles, does not form.
- Colloidal particles can bind to liquid interfaces with capillary energies that are a thousand times larger than their thermal energy.
- This phenomenon provides an effective way to stabilize emulsions and foams.
- It also changes the mobility status of the interface, and resists the interfacial flow/internal circulation at certain surface coverages.
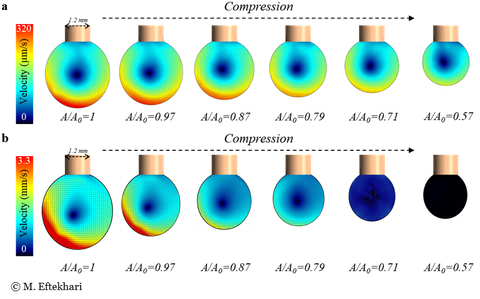
Internal circulation of a) surfactant- b) nanoparticle- laden bubbles under asymmetric shear flow and surface compression.
Synergistic effect of surfactants and nanoparticles on the interfacial properties
Motivation
Mixture of surfactants and particles are used in numerous industrial applications including oil recovery, froth flotation and wastewater treatment. Interactions between surfactants and particles can strongly influence the interfacial behavior of these systems.
1. Similarly charged surfactants and nanoparticles
Recent studies suggest that electrostatic repulsive forces play an important role in increasing the surface activity of surfactants in the presence of negatively charged nanoparticles.
Results
- The addition of nanoparticles decreases the surface tension of surfactant solution.
- The surface activity of the surfactants in the presence of similarly charged nanoparticles is mainly influenced by the increased ionic strength of the system.
- Surfactant molecules are influenced by electrostatic repulsion only at small distances from the particle surface where the electric potential is non-negligible.
2. Oppositely charged surfactants and nanoparticles
Despite the large body of work devoted to nanoparticles and surfactants of opposite charge, the key factors governing their interfacial behavior is not clearly identified.
Results
- Like surfactants, solid particles can create monolayers at the interfaces that change the interfacial behavior of the system.
- Surfactant to nanoparticle ratio is the dominant factor controlling the surface tension of the system.
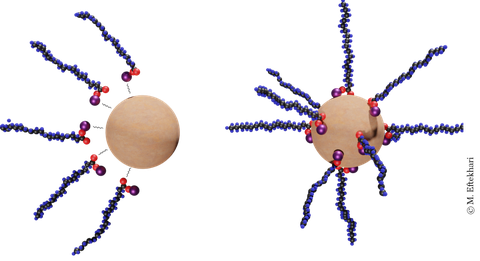
Schematic of nanoparticle-surfactant complex formation due to attractive electrostatic forces.
Publications
M. Eftekhari, K. Schwarzenberger, A. Javadi, K. Eckert: The influence of negatively charged silica nanoparticles on the surface properties of anionic surfactants: electrostatic repulsion or the effect of ionic strength? Physical Chemistry Chemical Physics, 22 (2020) 2238-2248
M. Eftekhari, K. Schwarzenberger, S. Heitkam, K. Eckert: Interfacial flow of a surfactant-laden interface under asymmetric shear flow. Journal of Colloid and Interface Science 599 (2021) 837-848
M. Eftekhari, K. Schwarzenberger, S. Heitkam, A. Javadi, A. Bashkatov, S. Ata, K. Eckert: Interfacial Behavior of Particle-Laden Bubbles under Asymmetric Shear Flow. Langmuir 37 (2021) 13244-13254