The Triply Deprotonated Acetonitrile Anion CCN3– Stabilized in a Solid
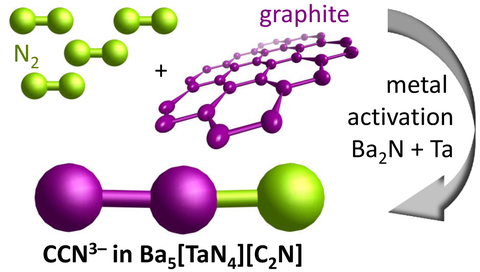
The first experimental realization of the long-sought triply deprotonated acetonitrile anion CCN3– succeeded by a remarkably simple solid state synthesis at 650 °C. CCN3– forms from the usually unreactive elements carbon and nitrogen owing to metal activation by Ba2N and Ta. This route is unparalleled in comparison with typical synthetic routes to N-containing organic compounds or carbon nitrides that employ reactive precursors like ammonia for C–N bond-formation.
Franziska Jach was able to stabilize the CO2 isoelectronic acetonitriletriide anion CCN3– in the bulk host framework of the Ba5[TaN4][C2N] nitridometalate. In a collaboration with Dr. Höhn and Prof. Grin’s working group at MPI CPfS and the working groups of Prof. Dr. Ruck and Prof. Dr. Brunner at TU Dresden, various methods were employed to comprehensively characterize this compound.
X-ray diffraction, NMR and vibrational spectroscopy in combination with quantum chemical calculations verified the molecular structure of the CCN3– anion and revealed a bonding situation with electron pairs shifted towards two double bonds in contrast to the acetonitrile molecule H3C–C≡N.
CCN3– is the most recent example of electron-rich anions being stabilized within a combined framework of a nitridometalate structure and alkaline-earth cations. With respect to earlier work on cyanamide anions CN22– and highly reduced cyano metalates such as [Co(CN)3]6–, alkaline-earth rich nitridometalates are a promising “cradle” for more highly charged anions.
This work was honored with the Hot Paper status and can be found in Angewandte Chemie:
F. Jach, S. I. Brückner, A. Ovchinnikov, A. Isaeva, M. Bobnar, M. Groh, E. Brunner, P. Höhn, and M. Ruck, Angew. Chem. 2017, 129, 2965–2968; Angew. Chem. Int. Ed. 2017, 56, 2919–2922.