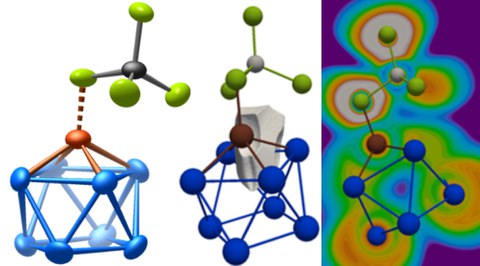
The intermetalloid cluster cation (CuBi8)3+
In the working group of Prof. Dr. Ruck, Maximilian Knies succeeded in the synthesis of two previously unknown compounds by the reaction of bismuth and bismuth trichloride with copper chloride in the ionic liquid [BMIm][Cl]⋅4 AlCl3:
(CuBi8)[AlCl4]2[Al2Cl7] and (CuBi8)[AlCl4]3.
Both crystal structures contain the intermetalloid cluster (CuBi8)3+. This is the first cluster that contains both bismuth and a 3d transition metal. Particularly impressive is the formation of the cluster at low pressure and low temperature, since binary compounds of copper and bismuth are otherwise observed only under extreme high pressure conditions.
In quantum-chemical calculations, seven shared electrons are found in multi-center bonds between the square-antiprismatic Bi82+ polycation and the copper(I) cation. The intermetalloid cluster can thus be described as a nine-atom, intermetalloid nido cluster with 22 skeletal electrons and C4v symmetry. Another description appears to be consistent as well: a η4 coordination of the square-antiprismatic Bi82+ polycation and further coordination of a chloride ion of a tetrahedral [AlCl4] group to the copper(I) cation, which leads to a total electron number of 18 for the copper(I) cation.
The work was honored with a front cover. The article can be found in Chemistry A European Journal:
M. Knies, M. Kaiser, A. Isaeva, U. Müller, T. Doert, M. Ruck, Chem. Eur. J. 2018, 24, 127–132.