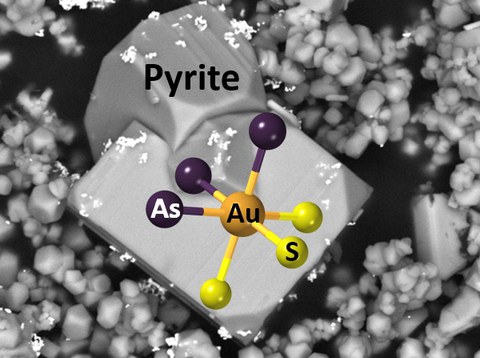
A mineral pump for gold in the Earth’s crust
To form an ore deposit, gold needs to be concentrated from a thousand up to a million times more than its average abundance in the Earth's crust (which is only about 1 mg per ton of rock). In nature, only very few minerals, namely arsenian pyrite and arsenopyrite, are known to present such enrichment factors for gold. However, despite the enormous implications, the state of this ‘invisible' gold and the cause of its entrapment in these sulfides remains one of the greatest mysteries in the history of the study of ore deposits.
An international interdisciplinary consortium of scientists was able to elucidate this mystery. They have shown the exact nature of gold intake by these minerals and revealed the fundamental mechanism that drives these ‘mineral pumps’ at the atomic-scale. By combining high-resolution experiments – carried out at the European Synchrotron facilities (ESRF) – and physical-chemical modeling, this team has discovered that gold enters these minerals with an oxidation state of +2. This is made possible by the occurrence of a redox reaction between fluid and mineral that allows binding gold to arsenic, leading to the formation of the atomic cluster AuAsnS6-n (Image). This universal gold-arsenic coupling mechanism explains how these sulfides can massively capture gold and release it later, controlling both concentration and distribution of gold in different types of hydrothermal deposits. This new conceptual model opens new perspectives for finding novel sources of gold and other precious and critical metals hidden in iron sulfide minerals, and for improving the processing and recycling of metal ores for our ‘metal-hungry’ society.