Hydroflux synthesis of the oxo hydroxoferrate K(2–x)Fe4O(7–x)(OH)x
The hydroflux synthesis is regarded as a combination of flux and hydrothermal synthesis. The reaction medium, the hydroflux, is an ultrabasic solution consisting of an approximately equimolar ratio of base and water. With this novel synthesis method, Ralf Albrecht in the working group of Prof. Dr. Ruck has succeeded in crystallizing the unknown oxo- hydroxoferrate K2–xFe4O7–x(OH)x (x ≈ 0.3) from a potassium hydroxide hydroflux.
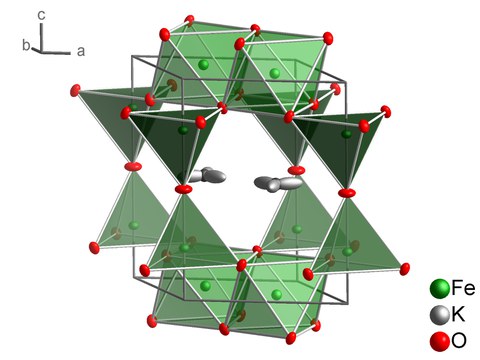
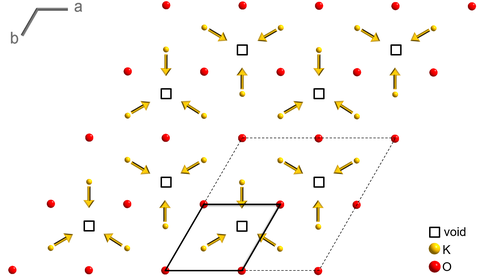
The crystal structure of this oxo-hydroxoferrate resembles a parking garage. The parking levels of this structure are composed of edge-sharing iron(III) octahedrons in a honeycomb network ∞2[Fe2O6]. Adjacent honeycomb nets are connected by pairs of vertex-sharing tetrahedra, the columns of the parking garage. The large voids between the honeycomb layers host mobile potassium ions, similar to cars in the parking garage. The charge difference of the potassium deficiency is balanced by hydroxide groups.
Above 2 K K2–xFe4O7–x(OH)x is antiferromagnetic and decomposes before the Néel temperature is reached. Upon heating to 350 °C the potassium ions start to order into a superstructure. Further heating to about 750 °C leads to a topotactical transformation of
K2–xFe4O7–x(OH)x into K1+x'Fe11O17
(β/β''-Aluminate structure type). This transformation requires a high mass transport of iron(III) polyhedra, as the honeycomb layers of K2–xFe4O7–x(OH)x have to be converted into a Kagome network. The final decomposition starts at 900 °C with formation of γ-Fe2O3, which then converts into the stable polymorph α-Fe2O3.
The article can be found in ChemistryOpen: