Dissolution of metal oxides in ionic liquid
The application of ionic liquids is a promising approach for the processing of metal oxides at low temperatures compared to conventional methods. This could be relevant for the mining and extraction of ores and minerals as well as recycling of (radioactive) waste, involving great energy savings.
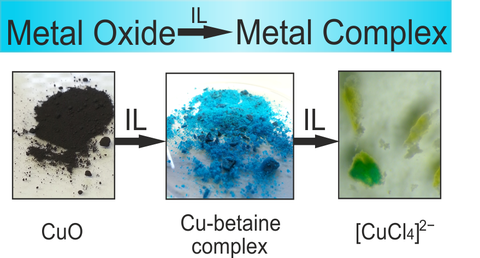
Janine Richter investigated the solubility of numerous metal oxides in the task-specific ionic liquid betainium bis(trifluoromethylsulfonyl)imide ([Hbet][NTf2]). These are the first comprehensive studies in water-free systems, which are relevant for further steps towards downstream chemistry or electrochemical metal deposition. By the addition of chloride anions, the solubility of many metal oxides could be increased, including not readily soluble representatives of this class of materials.
The crystal structure of the resulting complex compound in the system copper-[Hbet][NTf2], [Cu2(bet)4(NTf2)2][NTf2]2, was determined by X-ray crystallography. In this compound, coordination sites, free due to the sterical demands of betaine, are occupied by the weakly coordinating [NTf2]− anion. Subsequently, the extraction of copper from the blue crystals is possible by overlaying with the ionic liquid [P66614]Cl, giving the yellow complex [CuCl4]2− in dissolved form. This demonstrates a complete exchange of the oxygen-coordination sphere around copper by chlorido ligands, opening promising possibilities for downstream chemistry starting from metal oxides.
The work was honoured with the award of the ‘Dresdner Gesprächskreis der Wirtschaft und der Wissenschaft e. V.’ under the motto ‘Resources of the future – efficiency and sufficiency’. The article can be found in RSC Advances:

![[Cu2(bet)4(NTf2)2][NTf2]2](https://tu-dresden.de/mn/chemie/ac/ac2/ressourcen/bilder/forschungs-highlights/Fig1.png/@@images/084275ca-ec30-4f42-8dc2-f1ac02052832.png)