Reaction mechanism of the polyol process in the intermetallic Bi-Ni system
Typically, intermetallic phases are obtained in solid-state reactions or crystallization from melts, which are highly energy and time consuming. The polyol process takes advantage of low temperatures and short reaction times using easily obtainable starting materials. The formation mechanism of these intermetallic particles has received little attention so far, even though a deeper understanding should allow for better synthesis planning. In this study, Matthias Smuda in the working group of Prof. Dr. Michael Ruck therefore investigated the formation of BiNi particles in ethylene glycol in a microwave-assisted polyol process mechanistically.
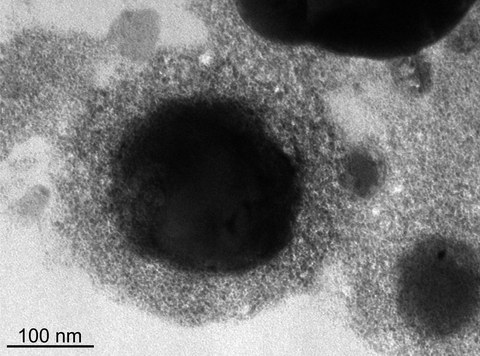
Bi-Ni core-shell intermediate
The coordination behavior in solution was analyzed using HPLC-MS and UV-Vis. Tracking the reaction with PXRD measurements, FT-IR spectroscopy and HR-TEM revealed a successive reduction of Bi3+ and Ni2+, leading to novel spherical core-shell structure in a first reaction step. Bi-particles are encased in a matrix of Ni‑nanoparticles of 2 nm to 6 nm in diameter and oxidation products of ethylene glycol. Step-wise diffusion of Ni into the Bi-particle intermediately results in the bismuth-rich compound Bi3Ni, which consecutively transforms into the BiNi phase as the reaction progresses.

Front Cover Chemistry Open
The impacts of the anion type, temperature and pH value were also investigated. An increase of the reaction temperature not only raises the reduction power of a given polyol, but also promotes the diffusion process leading to a significantly faster reaction. A higher pH value leads to the deprotonation of ethylene glycol. The newly formed glycolate has a higher coordination affinity towards the metal cations, promoting the reduction reaction. The anions of the salts have different binding affinities, which can favor or impede the reaction. A general series of reducibility can be given as AcO– > NO3– > Br– > Cl–.
The article was acknowledged with a front cover and can be found in ChemistryOpen:
Smuda, M., Damm, C., Ruck, M., Doert, T., ChemistryOpen 2020, 9(11), 1085–1094.
