Highlights at a glance
Unconventional Spin State in Pr3Fe3Sb7
A MINERAL PUMP FOR GOLD IN THE EARTH’S CRUST
Reaction mechanism of the polyol process in the intermetallic Bi-Ni system
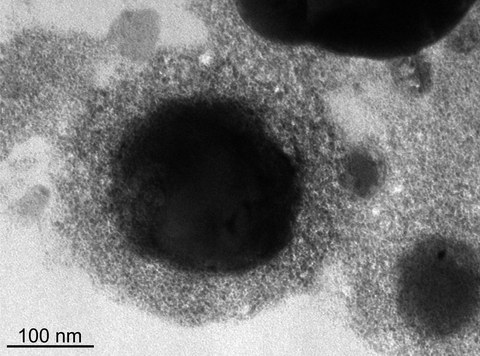
Bi-Ni core-shell intermediate

Bi-Ni core-shell intermediate