Forschung
Tissue Regeneration and Resolution of Inflammation
The immune system is the body's defense mechanism, safeguarding us against foreign substances and pathogens such as helminths, bacteria, and viruses. However, a combination of genetic, epigenetic, and environmental factors can sometimes cause the immune system to malfunction. This can lead to autoimmune diseases like rheumatoid arthritis and inflammatory bowel disease, or hypersensitivity reactions such as allergic asthma. These chronic conditions necessitate lifelong medication adjustments for patients, imposing substantial health and economic burdens on both individuals and society.
Our research aims to explore cellular and molecular signaling pathways that contribute to misguided immune responses, leveraging this knowledge to promote the resolution of chronic diseases and restore tissue homeostasis. A key focus of our research is to understand how an inflammatory microenvironment in various tissues alters the function of innate immune cells at the genetic, metabolic, and proteomic levels. As part of our translational approach, we integrate studies on genetically modified mice with analyses of human tissues and cells, employing cutting-edge omics and imaging techniques. Our current research topics include:
Unravelling the Regulatory Role of Eosinophils in Resolution of Inflammation and Tissue Regeneration
We are exploring the often overlooked homeostatic role of eosinophils in resolving inflammation and regenerating tissue. Eosinophils are typically regarded as downstream effector cells in inflammatory processes due to their ability to secrete granule-derived pro-inflammatory mediators, mainly in the context of allergic responses and helminth infections. However, eosinophils also store growth factors, anti-inflammatory cytokines, and resolvins, enabling them to contribute to the resolution of inflammation and tissue regeneration. Notably, eosinophils are abundantly present in various tissues such as bone marrow, spleen, uterus, adipose tissue, small intestine, and skin, where they likely perform homeostatic tasks. In line with the hygiene hypothesis, autoimmune diseases driven by Type-1 and Type-17 immunity, such as rheumatoid arthritis, are counteracted by Type-2 immune responses associated with high eosinophil levels (Chen, Andreev et al., Nat Commun. 2016). However, the underlying mechanisms are still unknown.
Our previous work has demonstrated the existence of a regulatory eosinophil population in the joints capable of resolving inflammatory arthritis. These synovial regulatory eosinophils have distinct genetic, proteomic, and metabolic signatures compared to their counterparts activated by allergic asthma in the lungs (Andreev, Liu et al., Ann Rheum Dis. 2021). Additionally, eosinophils located in the bone marrow can maintain healthy bone metabolism by counteracting excessive bone resorption by osteoclasts (Andreev et al., Nat Commun. 2024). These data suggest that the tissue microenvironment might reshape eosinophils from a regulatory to an inflammatory state in conditions such as asthma. In the future, we aim to determine whether eosinophils acquire specific tissue-dependent phenotypes through epigenetic and metabolic adaptations, focusing on their capacity to secrete certain mediators and their interactions with cells from the myeloid lineage, such as macrophages and osteoclasts. Our long-term goal is to reprogram eosinophils toward a tissue-protective, pro-resolving phenotype to promote tissue healing. This approach could benefit not only arthritis but also inflammatory conditions triggered by an aggressive eosinophil phenotype, such as asthma, colitis, and dermatitis.
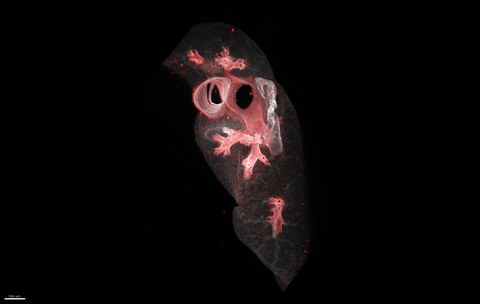
Light sheet fluorescence microscopy of eosinophils (red) in lung tissue.
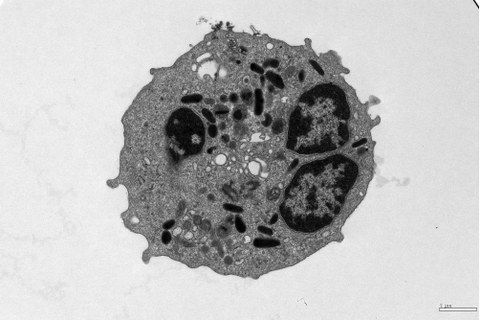
Transmission electron microscopy of an eosinophil isolated from blood.
The Role of Heme Peroxidases as Pro-resolving Agents in Inflammatory Arthritis
The objective of this project is to uncover a novel immune regulatory function of heme peroxidases in rheumatoid arthritis. These peroxidases are produced in high amounts by granulocytes and can be found in arthritic joints. Traditionally, their role has been to generate reactive oxygen species as a defense mechanism against invading pathogens. However, recent research suggests that heme peroxidases have a much more intricate role in the immune system, possessing not only pro-inflammatory properties but also various immune-modulatory effects.
The main focus of this project is to investigate how heme peroxidases may promote the transition of classical pro-inflammatory macrophages to alternatively activated macrophages with anti-inflammatory qualities, thereby facilitating the resolution of inflammatory arthritis.
The Effects of Alarmins on Bone Regeneration
Bone remodeling is the primary metabolic process that maintains a stable and functional skeleton in adulthood. Approximately 10% of all bones in the human body are remodeled each year. This process is understood as a cycle. Bone damage, such as microfractures, triggers the recruitment of osteoclasts. These cells then remove the damaged bone. It is crucial to stop this bone resorption process to enable the regeneration of the bone matrix by osteoblasts. However, an imbalance in this process in favor of osteoclast-mediated bone resorption can often lead to pathological bone loss, as seen in aging, postmenopausal osteoporosis, and autoimmune diseases. To date, it is unclear how osteoclasts are recruited to the damaged bone. One potential mechanism is the recognition of alarmins released during cell damage. Since osteoclasts develop from the monocyte/macrophage lineage, they also express the corresponding pattern recognition receptors on their surface. Indeed, we could demonstrate that the DAMP-sensing receptor macrophage-inducible C-type lectin (Mincle) is involved in osteoclast-mediated bone resorption (Andreev et al., J Clin Invest. 2020).
In this project, we plan to investigate the morphological, cellular, and molecular effects of microfractures and large-scale bone injuries, such as traumatic fractures, using newly developed omics platforms and imaging techniques. Overall, these experiments aim to decipher the role of alarmins during bone regeneration, beyond their typically defined pro-inflammatory properties, as regulators of bone resorption by osteoclasts and bone formation by osteoblasts.
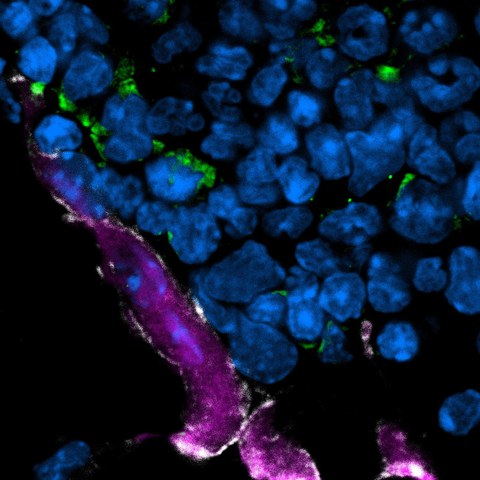
Confocal laser scanning microscopy of bone tissue showing proximity of polynucleated (DAPI in blue) osteoclasts (purple) with eosinophils (green).
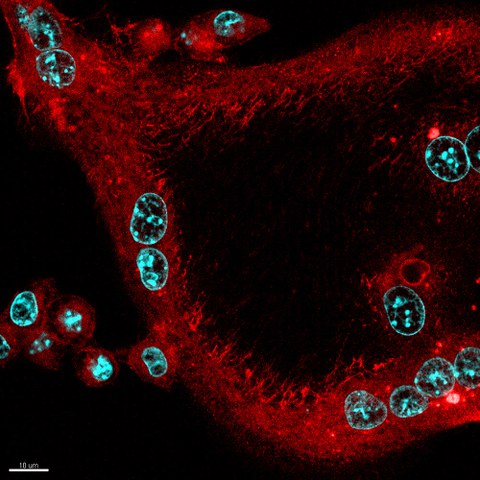
Confocal laser scanning microscopy of f-actin in in vitro differentiated polynucleated (DAPI in cyan) osteoclast (red)
