Research
Glycosaminoglycan-based cell-instructive hydrogels
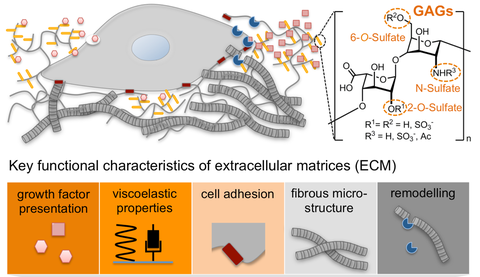
Implementing a theory-driven design strategy, we have explored the incorporation of glycosaminoglycans (GAGs) in biohybrid polymer hydrogels for the biomimetic modulation of growth factor signals, along with other cues of extracellular matrices (ECM). Applications of customized GAG-based materials include inflammation-modulating wound dressings, cell replacement-supporting cryogel microcarriers for neurodegenerative diseases and metabolic disorders as well as gel matrices to stimulate embedded hematopoietic stem cells ex vivo. The materials were furthermore successfully used to induce tubulogenesis of human vascular endothelial cells as well as of human renal and mammary epithelial cells, supported the formation of immunotherapeutic organoids and enabled the ex vivo analysis of tumor vascularization and acute myeloid leukemia in response to treatment.
Adaptive hemocompatible coatings
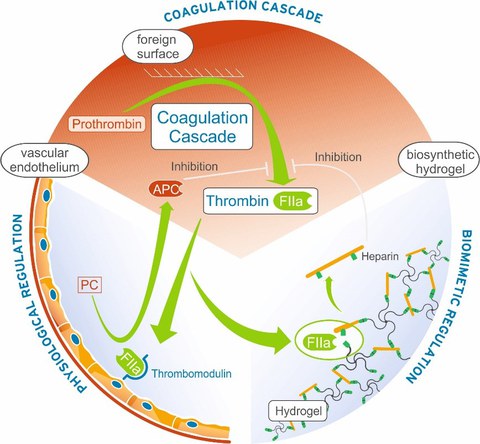
We have demonstrated that bio-responsive polymer architectures can empower medical therapies by engaging molecular feedback-response mechanisms resembling the homeostatic adaptation of living tissues to varying environmental constraints: A blood coagulation-responsive hydrogel system was shown to deliver heparin in amounts triggered by the environmental levels of thrombin, the key enzyme of the coagulation cascade, which--in turn- becomes inactivated due to released heparin. The bio-responsive hydrogel quantitatively quenches human blood coagulation over several hours in the presence of pro-coagulant stimuli. Release analogously triggered by the "early" coagulation factors Xa or XIIa enhanced the efficiency of the released anticoagulant, and silver-containing hydrogel layers provided a combination of hemocompatibility and long-term antiseptic capacity.
Biomimetic control of wetting and adhesion
We have explored the cuticular morphology and chemistry of liquid-repellent cuticula of springtails (Collembola), arthropods living in the soil, and derived general design criteria for robust omniphobic surfaces. The resistance of the cuticle against wetting and its mechanical stability were shown to depend on a combination of overhanging cross-sections and their arrangement in a self-supporting comb-like pattern, features that we could successfully translate into robust omniphobic polymer membranes that can be even applied to curved surfaces.
The aims of our ongoing and future research are
- bioinspired approaches to adaptively minimize bacterial adhesion
- immunomodulatory polymer materials to support wound healing
- multiphasic morphogenetic polymer matrices for in situ tissue engineering and for organoid cultures