Anion exchange of PX3 (X = Cl, Br, I) observed in ionic liquids
Red phosphorus is a network-like structure of phosphorus atoms that is very stable and, thus, undergoes just a few reactions under normal conditions. However, previous studies have shown that red phosphorus reacts with iodine in ionic liquid (ILs) already at room temperature to produce various phosphorus iodides (PI3, P2I4, [P2I5]+) (Groh et al., Eur. J. Inorg. Chem. 2015, 3991–3994.). In a cooperation of the working groups of Prof. Dr. Ruck and Prof. Dr. Brunner (TU Dresden) detailed nuclear resonance and vibration spectroscopic investigations were conducted to obtain a better understanding of this dissolution process.
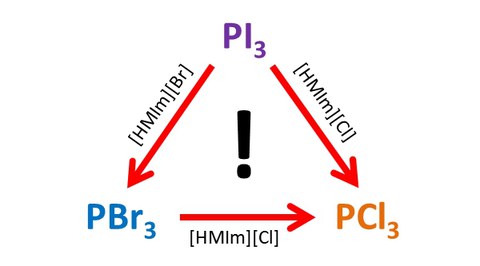
In their investigations, Julia Pallmann and Alexander Wolff found that various dissolved PX3 (X = Cl, Br, I) molecules are formed immediately in the IL after the initial reaction of red phosphorus with iodine. The actual chemical composition of these molecules strongly depends on the anion present in the IL. For example, the formed PI3 molecules are transformed immediately into PBr3 using the IL [HMIm][Br] and into PCl3 using the IL [HMIm][Cl]. All reactions take place already at room temperature. It is thus possible to adjust the reactive species by the choice of the IL, which might become crucial for possible applications.
The work was honored with a front cover. The article can be found in the Journal of General and Inorganic Chemistry:
A. Wolff, J. Pallmann, E. Brunner, T. Doert, M. Ruck, Z. Anorg. Allg. Chem. 2017, 643, 20–24.