Forschung
Sehbehinderungen und Blindheit, die durch die Degeneration der lichtempfindlichen Photorezeptoren und/oder des unterstützenden retinalen Pigmentepithels (RPE) verursacht werden, wie bei der altersbedingten Makuladegeneration (AMD) oder Retinitis pigmentosa, stellen eine der Hauptursachen für Behinderungen in den Industrieländern dar, wobei es derzeit keine wirksamen Behandlungsmethoden gibt. Unsere experimentelle Arbeit konzentriert sich auf die Entwicklung von zellbasierten Strategien zum Ersatz verlorener Zellen in der Netzhaut durch die Transplantation von Photorezeptoren und RPE-Zellen.
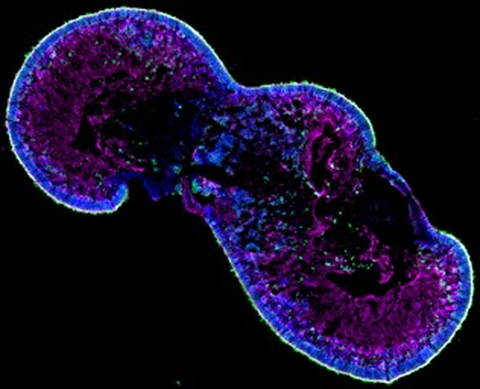
Eine in vitro expandierbare Zellquelle für die Generierung von transplantierbaren Photorezeptoren und RPE-Zellen ist für eine Umsetzung in die klinische Anwendung unerlässlich. Daher werden Photorezeptoren enthaltende retinale Organoide und RPE-Zellen, die aus induzierten pluripotenten Stammzellen gewonnen werden, derzeit für Transplantationsstudien in präklinischen Netzhautdegenerationsmodellen verwendet, um ihr Potenzial zur funktionellen Reparatur zu nutzen.
Interessanterweise konnten wir zeigen, dass Spender-Photorezeptoren aus der Maus nicht strukturell in das Wirtsnetzhautgewebe integriert werden, sondern sich stattdessen an der Transplantationsstelle zwischen der Photorezeptorschicht und dem RPE, dem so genannten subretinalen Raum, aufhalten und zytoplasmatisches Material mit den Wirts-Photorezeptoren austauschen, was eine Neuinterpretation früherer Transplantationsstudien mit Maus-Photorezeptoren impliziert. Darüber hinaus haben wir in neueren Arbeiten nachgewiesen, dass zytoplasmatischer Materialtransfer auch zwischen Zapfen- und Stäbchenphotorezeptoren in der normalen Säugetiernetzhaut stattfindet, möglicherweise über zytoplasmatische Röhren, die zwischen den beteiligten Zellen gebildet werden.
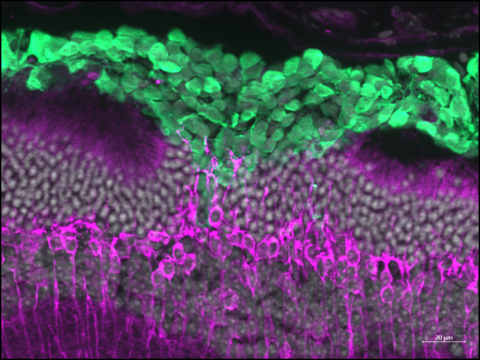
Im Gegensatz zu transplantierten Mäusephotorezeptoren haben wir vor kurzem die strukturelle Integration von menschlichen Photorezeptoren, die aus retinalen Organoiden gewonnen wurden, in ein Mausmodell für Zapfendegeneration nachgewiesen. Die eingepflanzten menschlichen Photorezeptoren bildeten innere und äußere Segmente und scheinen mit den Bipolarzellen des Wirts eine Synapse zu bilden, die die Reparatur der photopischen Wahrnehmung ermöglicht, die den retinalen Ganglienzellen signalisiert wird. Wichtig ist, dass die Interaktion mit Müller-Glia und Neuronen zweiter Ordnung des Wirts für die ordnungsgemäße Reifung und Funktion der Spender-Photorezeptoren wesentlich ist. Die Identifizierung der zellulären und molekularen Mechanismen, die die strukturelle und funktionelle Integration von Spender-Photorezeptoren ermöglichen, ist eine äußerst wichtige Aufgabe für die Entwicklung von Zellersatztherapien für die klinische Anwendung. Daher untersucht unser Labor derzeit die grundlegenden Voraussetzungen der Spenderzellen sowie die Wirtsumgebung, um optimale Bedingungen für einen funktionellen Photorezeptor- und RPE-Ersatz in der degenerativen Netzhaut von Säugetieren zu definieren.