Research
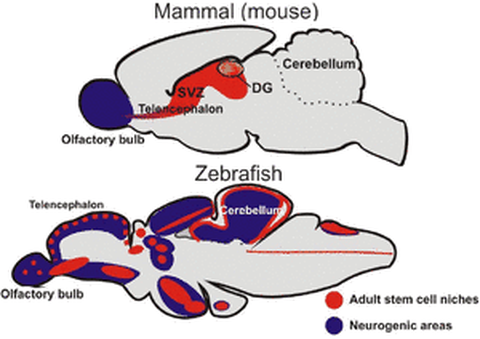
In contrast to mammals, the central nervous system (CNS) of adult zebrafish retains a large number of active neural stem/progenitor cells producing numerous new neurons of different subtypes in discrete spatial domains. Moreover, the adult zebrafish CNS has a spectacular ability to regenerate after severe lesions. Combined with well-developed genetic and molecular biology tools, it therefore provides an ideally tractable system for understanding recruitment of stem cells during normal homeostasis and in the context of regeneration.
Using various CNS lesion paradigms, transgenesis, Cre/loxP technology and next generation sequencing we investigate which genes and pathways regulate adult neural stem cell activity and their ability to repair damage. We also address how the distribution of organizer-associated signalling molecules is propagated in the embryonic and adult CNS controlling the formation and maintenance of compartment boundaries.
Our studies will provide clues on how CNS regeneration can be stimulated also in mammalian brains and pave the way for stem cell based regenerative therapies against acute as well as chronic brain injury.
1. Exploring the molecular cues driving zebrafish central nervous system regeneration
Zebrafish, in striking contrast to mammals, can functionally regenerate complex body structures, including significant portions of the central nervous system. Thus, understanding this regenerative capacity of zebrafish nervous system is of great therapeutic significance to treat neural diseases in humans. We are investigating the genes and the signaling pathways involved in the regeneration of the adult zebrafish central nervous system focusing on the role of neurogenic progenitor cells by using different injury, transcriptional profiling and misexpression methods.
Scientists involved: Sibel Aktay, Avinash Chekuru, Dilce Gozuyasli, Dr. Caghan Kizil, Nikos Kyritsis, Jan Martin Pidun, Weronika Welnicz
Related publications:
Kizil C, Brand M (2011) Cerebroventricular Microinjection (CVMI) into Adult Zebrafish Brain Is an Efficient Misexpression Method for Forebrain Ventricular Cells. PLoS ONE 6(11): e27395. doi:10.1371/journal.pone.0027395.
Kroehne V, Freudenreich D, Hans S, Kaslin J, Brand M. (2011) Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development. 138(22):4831-41.
Antos CL, Brand M (2011) Regeneration of Organs and Appendages in Zebrafish: A Window into Underlying Control Mechanisms. In: Encyclopedia of Life Sciences, John Wiley & Sons, Ltd: Chichester www.els.net
Kizil C, Kaslin J, Kroehne V, Brand M (2011). Adult neurogenesis and brain regeneration in zebrafish. Dev Neurobiol. 72(3):429-461.
Kizil C, Dudczig S, Kyritsis N, Machate A, Blaesche J, Kroehne V, Brand M. (2012) The chemokine receptor cxcr5 regulates the regenerative neurogenesis response in the adult zebrafish brain. Neural Dev. 7:27.
Kizil C, Kyritsis N, Dudczig S, Kroehne V, Freudenreich D, Kaslin J, Brand M. (2012) Regenerative neurogenesis from neural progenitor cells requires injury-induced expression of Gata3. Dev Cell. 23(6):1230-7.
Kyritsis N, Kizil C, Zocher S, Kroehne V, Kaslin J, Freudenreich D, Iltzsche A, Brand M. (2012) Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science. 338(6112):1353-6.
Kaslin J, Kroehne V, Benato F, Argenton F, Brand M. (2013) Development and specification of cerebellar stem and progenitor cells in zebrafish: from embryo to adult. Neural Dev. 8(1):9.
Kizil, C., Iltzsche, A., Kaslin, J., Brand, M. (2013) Micromanipulation of Gene Expression in the Adult Zebrafish Brain Using Cerebroventricular Microinjection of Morpholino Oligonucleotides. J. Vis. Exp., (75), e50415.
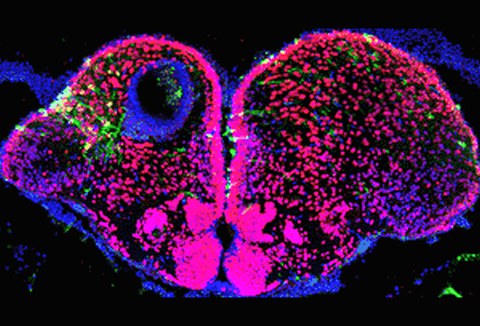
2. Characterization of neurogenic stem cell niches in zebrafish adult brain
Adult neurogenesis is a conserved trait of all vertebrates studied. It has been investigated in detail in rodents, but very little is known about the composition of neurogenic niches and the cellular nature of progenitors in non-mammalian vertebrates. To understand the cellular characteristics of the progenitor domains, we are investigating the molecular regulatory networks of the neurogenic progenitor cells in different regions of the adult zebrafish brain.
Scientists involved: Vanessa Carlos, Avinash Chekuru, Dr. Heiner Grandel, Dr. Stefan Hans, Dr. Caghan Kizil, Nikos Kyritsis
Related publications:
Grandel, H., Kaslin, J., Ganz, J., Wenzel, I., and Brand, M. (2006). Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Developmental Biology, 295(1):263-277.
Kaslin J, Ganz J, Brand M. (2008). Proliferation, neurogenesis and regeneration in the non-mammalian vertebrate brain. Philos Trans R Soc Lond B Biol Sci. 29;363 (1489):101-122.
Kaslin, J., Ganz, J., Geffarth, M., Grandel, H., Hans, S. and Brand M. (2009). Stem Cells in the Adult Zebrafish Cerebellum: Initiation and Maintenance of a Novel Stem Cell Niche. J Neuroscience, 29(19):6142-53.
Ganz J., Kaslin J., Hochmann S., Freudenreich D., Brand M. (2010) Heterogeneity and Fgf dependence of adult neural progenitors in the zebrafish telencephalon. Glia, 58(11):1345-1363
Ganz J, Kaslin J, Freudenreich D, Machate A, Geffarth M, Brand M. (2011) Subdivisions of the adult zebrafish subpallium by molecular marker analysis. J Comp Neurol. 520(3):633-55
Grandel H, Brand M. Comparative aspects of adult neural stem cell activity in vertebrates. Dev Genes Evol. 2013 Mar;223(1-2):131-47
3. Characterization of a novel zebrafish model for Parkinson’s disease
Neurodegenerative diseases such as Alzheimer’s or Parkinson’s are a major public health concern. Despite decades of research, success in reproducing human neurodegeneration in animal models such as mice has been incomplete rasing the need for novel and complementary approaches. Given the twofold advantage of: 1) being a vertebrate; and 2) being a relatively simple organism where to study comprehensive pathology in vivo, in recent years the zebrafish has turned out a promising model to understand human diseases, including motor disorders and neuropsychiatric diseases. We have generated and are currently characterizing a zebrafish mutant with features reminiscent of human Parkinson’s disease (PD). Our aim is to formulate novel hypotheses for PD aetiopathology and/or possible clinical applications.
Scientists involved: Dr. Stefan Hans, Stefano Suzzi
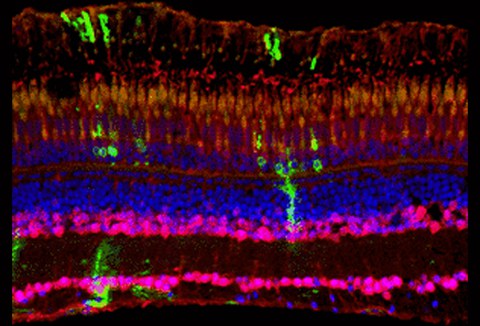
4. Regeneration of the adult zebrafish retina
Since photoreceptor cell loss during adulthood is one of the major causes for blindness in humans, it is of huge interest to investigate retinopathy studies in regenerating model organisms. Unlike mammals, zebrafish gain a tremendous ability to regenerate the retina. We analyze the importance of Fgf signaling in the developing and adult retina. We were able to show that Fgf signaling is required for photoreceptor maintenance. Using different lesion paradigms and the Cre/LoxP technology we are interested in lineage tracing of various cell populations in the regenerating retina and elucidating the underlying mechanisms.
Scientists involved: Karina Geurtzen, Dr. Veronika Kuscha, Anke Weber
Related publications:
Picker, A. and Brand, M. (2005): Fgf-signals from a novel signaling center determine axial patterning of the prospective neural retina. Development 132 (22):4951-4962
Picker A, Cavodeassi F, Machate A, Bernauer S, Hans S, Abe G, Kawakami K, Wilson SW, Brand M. (2009) Dynamic coupling of pattern formation and morphogenesis in the developing vertebrate retina. PLoS Biol. Epub 2009 Oct 13.
Hochmann S, Kaslin J, Hans S, Weber A, Machate A, Geffarth M, Funk R. H. W., Brand M. (2012) Fgf Signaling is Required for Photoreceptor Maintenance in the Adult Zebrafish Retina. PLoS ONE 7(1): e30365.
5. In vivo molecular measurements of FGF8 morphogen propagation in the zebrafish embryo
Morphogens are signaling molecules which travel across significant distances through the complex network of extracellular matrix to mediate specific cellular responses. Using single molecule Fluorescence Correlation Spectroscopy FCS), we have demonstrated that Fgf8 moves through the extracellular matrix (ECM) by free diffusion and forms a gradient by receptor-mediated endocytosis. Using the biophysical technique of FCS we are further trying to understand how the complex interplay of cell-surface receptors and ECM components regulate the propagation of Fgf8 in a developing zebrafish embryo.
Scientists involved: Mansi Gupta
Related publications:
Scholpp S, Brand M.(2004). Endocytosis controls spreading and effective signaling range of Fgf8 protein. Curr Biol.14(20):1834-41.
Yu, S.R., Burkhardt, M., Nowak, M., Ries, J., Petrášek, Z., Scholpp, S., Schwille, P. and Brand, M. (2009). FGF8 morphogen gradient is formed by a source-sink mechanism with freely-diffusing molecules. Nature, 461(7263):533-6.
Ries, J. , Yu, S. R., Burkhardt, M., Brand, M. and Schwille, P. (2009). Modular scanning FCS quantifies ligand-receptor interactions in live multicellular organisms. Nature Methods.
Nowak M., Yu SR, Mansi G, Machate A, Brand M (2011) Interpretation of the Fgf8 morphogen gradient is regulated by endocytic trafficking. Nature Cell Biology 13(2):153-158.
Boldajipour, B, Tarbaschevicha K, Doitsidou M, Laguric C, Yu RZ, Riese J, Dumstrei K, Dörries J, Messerschmidt E-M, Schwille P, Brand M, Lortat-Jacobc H, Raz E (2011). A role for biased receptor recognition in chemokine subfunctionalization and evolution. Development 138: 14: 2909-2914.
Müller P, Rogers KW, Yu SR, Brand M, Schier AF. (2013) Morphogen transport. Development. 140(8): 1621-38..
Bökel C, Brand M. (2013) Generation and interpretation of FGF morphogen gradients in vertebrates. Curr Opin Genet Dev.
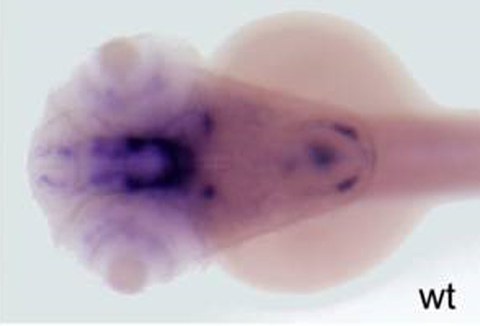
6. Patterning and the cell movements of the embryonic midbrain-hindbrain boundary in zebrafish
Boundary formation is essential for restricting the intermingling of cells with different developmental potentials, and it also allows formation of local signaling centers. It is the key fundamental process that coordinates growth and patterning of the embryo. Understanding the formation and maintenance of boundaries during growth and morphogenesis is essential for broadening our knowledge on basic developmental biology. We are investigating the molecular mechanisms that regulate boundary formation using various zebrafish mutants.
Scientists involved: Dr. Gokul Kesevan
Related publications:
Langenberg, T.L. and Brand, M. (2005). Lineage restriction maintains a stable organizer cell population at the zebrafish midbrain-hindbrain boundary. Development, 132(14):3209-3216.
Rhinn, M., Lun, K., Luz, M., Werner, M., Brand, M. (2005). Positioning of the midbrain-hindbrain boundary organizer through global posteriorisation of the neuroectoderm mediated by Wnt8 signaling. Development 132, 1261-1272.
Rhinn, M., Picker, A., and Brand, M. (2006). Global and local mechanisms of forebrain and midbrain patterning. Curr Opin Neurobiology 16, 1-8.
Dahmann, C, Oates, AC, Brand, M (2011) Boundary formation and maintenance in tissue development. Nature Review Genetics, 12(1):43-55.
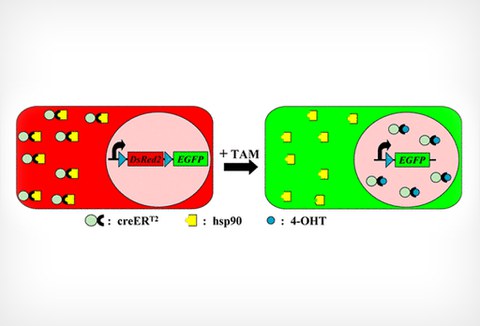
7. Developing site-specific recombinase technology for adult zebrafish brain
We demonstrated that using transposon-mediated transgenesis, the site-specific recombinase Cre is highly efficient and spatiotemporally controllable in zebrafish. Our work provides the opportunity to develop recombinase-mediated techniques for genomic manipulations including conditional knockout alleles, temporal and spatial overexpression studies and bona fide lineage tracing.
Scientists involved: Dr. Stefan Hans, Peggy Jungke
Related publications:
Hans, S., Kaslin, J., Freudenreich, D., and Brand, M. (2009). Temporally-controlled Site-specific Recombination in Zebrafish. PLoS ONE. 2009;4(2):e4640.
Hans, S., Freudenreich, D., Geffarth, M., Kaslin, J., Machate, A. and Michael Brand (2011). Generation of a non-leaky heat shock-inducible Cre line for conditional Cre/lox strategies in zebrafish. Developmental Dynamics 240(1):108-115.
Jungke P, Hans S, Brand M. (2013) The Zebrafish CreZoo: An Easy-to-Handle Database for Novel CreER(T2)-Driver Lines. Zebrafish.
8. Formation of inner ear neurons during development and adulthood
The vertebrate inner ear provides hearing and balance due to a complex arrangement of sensory hair cells, supporting cells and neurons. All these cell types derive from the otic placode, a transient ectodermal thickening adjacent to the developing hindbrain. Following induction, the otic placode develops into a vesicle where hair cells and neuronal precursors are born that require the activation of the proneural proteins Atoh1 and Neurog1. We are interested in the genes and signaling pathways controlling sensory and in particular neuronal precursor formation upstream of the proneural proteins during development and adulthood.
Scientists involved: Dr. Stefan Hans
Related publications:
Léger S, Brand M. (2002); Fgf8 and Fgf3 are required for zebrafish ear placode induction, maintenance and inner ear patterning. Mech Dev. 119: 91-108.
Hans, S., Liu, D. and Westerfield, M. (2004); Pax8 and Pax2a function synergistically in otic specification, downstream of the Foxi1 and Dlx3b transcription factors. Development 131, 5091-5102.
Hans, S., Christison J., Liu D. and Westerfield, M. (2007); Fgf-dependent otic induction requires competence provided by Foxi1 and Dlx3b. BMC Dev Biol. 19, 7:5.
Hans, S. and Westerfield, M. (2007); Changes in retinoic acid signaling alter otic patterning. Development 134, 2449-58
Hans S, Irmscher A, Brand M. (2013) Zebrafish Foxi1 provides a neuronal ground state during inner ear induction preceding the Dlx3b/4b-regulated sensory lineage. Development. 140(9):1936-45.