Ader Lab
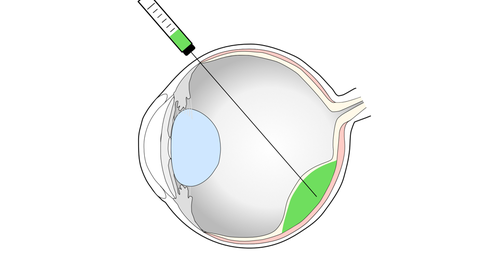
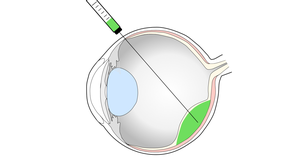
Vision impairment and blindness caused by the degeneration of the light sensitive photoreceptors and/or the supporting retinal pigment epithelium (RPE), as in age-related macular degeneration (AMD) or retinitis pigmentosa, represents one of the prime causes for disability in industrialized countries, with no effective treatments currently established. Our experimental work focuses on the development of cell-based strategies to replace lost cells in the retina by the transplantation of photoreceptors and RPE cells.
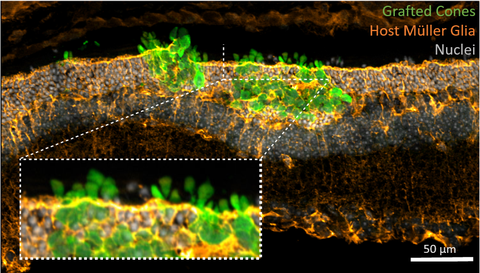
For this, photoreceptor-containing retina organoids and RPE are generated from human induced pluripotent stem cells (iPSC) and enriched for transplantation into pre-clinical retinal degeneration models. Human photoreceptor transplants show extensive structural integration into the degenerated retina, develop a mature morphology including inner- and outer-segments as well as synaptic contacts to second order neurons leading to functional changes upon light stimulation. Additionally, transplanted RPE cells generate polarized monolayers in a RPE-depletion mouse model providing barrier function and phagocytosis of outer segements. We now assess the mechanism of cell integration and function, and combine photoreceptor and RPE replacement by a sequential co-transplantation approach.
 © Ader Lab, CRTD
© Ader Lab, CRTD
Our research
Vision impairment and blindness caused by the degeneration of the light sensitive photoreceptors and/or the supporting retinal pigment epithelium (RPE), as in age-related macular degeneration (AMD) or retinitis pigmentosa, represents one of the prime causes for disability in industrialized countries, with no effective treatments currently established. Our experimental work focuses on the development of cell-based strategies to replace lost cells in the retina by the transplantation of photoreceptors and RPE cells.
In recent years we provided proof-of-concept studies for photoreceptor survival and maturation following retinal transplantation into mouse models of retinal degeneration, defined the optimal developmental stage for transplantable photoreceptors, identified cell surface markers for efficient enrichment of rod photoreceptors, and demonstrated daylight vision repair following transplantation of cone-like photoreceptors into a mouse model with degenerated cones.
An in vitro expandable cell source for the generation of transplantable photoreceptors and RPE cells will be mandatory for the translation towards clinical application. Therefore, photoreceptor-containing retina organoids and RPE from pluripotent stem cells (mouse and human embryonic and induced pluripotent stem cells) are currently used for transplantation studies in pre-clinical retinal degeneration models to access their potential for functional repair.
Recent results provide evidence that donor photoreceptors actually do not structurally integrate into host retinal tissue, that still contains endogenous photoreceptors, but instead reside between the photoreceptor layer and the RPE, the so called sub-retinal space, and exchange intracellular material with host photoreceptors. Our results contradict the common view that transplanted photoreceptors migrate and integrate into the photoreceptor layer of recipients and therefore imply a re-interpretation of previous photoreceptor transplantation studies. Actually, the observed interaction of donor with host photoreceptors may represent an unexpected mechanism for the treatment of blinding diseases in future cell therapy approaches.