Research
PREVIOUS AND CURRENT RESEARCH
A research highlight that laid the groundwork for our lab was the first evidence for the experimental stimulation of a regenerative response in the mammalian retina: The major type of glia cells in the retina, Müller glia, may produce new neurons (regeneration) in the damaged adult mouse retina in vivo (Karl et al PNAS 2008). However, whether a regenerative response can be elicited in human retina is still unknown.
It is well known that glia at least may gain other functions with beneficial but also detrimental consequences, which either prevent and protect or cause and contribute to retinal diseases, respectively.The regulatory mechanisms that facilitate and limit regeneration, and those controlling other glia functions in pathologies are also still incompletely understood.
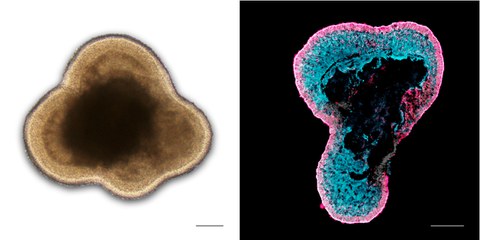
Left: Brightfield microscopy image of a living human retinal organoid in cell culture; Right: Immunostained image of a human retinal organoid section labeled for photoreceptors (RCVRN, magenta) and Müller glia (RLBP1, cyan)
Thus, a fundamental question that we are exploring in our research is whether neural scar formation and pathologic function of glia and neurons – highly relevant for AMD and other retinal pathologies in humans, might be part of a misregulated regeneration response or separate entities.
Further, to model neuronal degeneration and regeneration in the human retina, we develop and optimize methods to generate human retinas in the laboratory: Pluripotent stem cells can be differentiated in cell culture to develop 3D organ-like structures – called retinal organoids. We currently apply mouse and human retinal organoids to effectively reproduce retinal pathologic processes, and particularly focus on cone and rod photoreceptor dystrophies and glial pathologies.
FUTURE PROJECTS AND GOALS
Our current overall objectives are to develop human stem cell derived neural organoid and cell systems, specifically for the retina, to apply these to understand mechanisms of neuronal degeneration and regeneration, and thereby to find new therapeutic strategies for a broad patient spectrum, particularly, age-related macular degeneration (AMD).
Interested in detailed aims? Stay tuned!
