Research
Organization and Dynamics of Plasma Membrane Protrusions: From Biochemistry and Cell Biology to Tissue Engineering, Regenerative Therapy and Diseases
We studied the organization and dynamics of the plasma membrane, in particular cellular protrusions, as well as the molecular and cellular mechanisms of intercellular communication. We use three distinct cellular models; stem (cancer stem) cells, differentiated epithelial cells and photoreceptor cells, which share the expression of the cholesterol-binding pentaspan membrane glycoprotein Prominin-1 (alias CD133). We have identified and/or cloned this protein and other prominin family members in various species (e.g., human, mouse, chick, zebrafish, axolotl, drosophila). This molecule has generated enormous interest in the medical field because its expression on the cell surface allows the immuno-isolation of somatic stem cells from various tissues including the brain, blood, kidney and prostate. Prominin-1 highlights cancer-initiating cells. Recessive and dominant mutations in the PROM1 gene are associated with retinal diseases. In our investigation, we use Prominin 1 as a double marker of plasma membrane protrusions (e.g., microvilli, cilia) and cholesterol-based membrane microdomains (called lipid rafts).
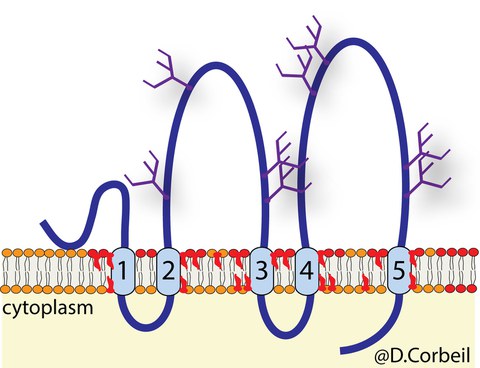
Schematic representation of Prominin-1 (CD133, PROM1)
Organization and Dynamics of the Plasma Membrane of Epithelial Cells
Prominin-1 shows a strong preference for highly curved membranes such as those found in microvilli and the primary cilium at the apical plasma membrane of terminally differentiated epithelial cells. By following its intracellular traffic, we have shown that the apical membrane is composed of distinct membrane microdomains. For example, ganglioside GM1–enriched lipid rafts that contain Prominin-1 are found in microvilli, while those enriched in GM3 are associated with the planar portion of the apical membrane. The primary cilium contains both gangliosides. These membrane microdomains might organize the structure and perhaps the functions of the apical membrane. Indeed, this membrane is highly dynamic since extracellular vesicles containing Prominin-1 bud from the tips of microvilli and/or primary cilium. The interactions of Prominin-1 with numerous cytoplasmic protein partners that regulate the structure of microvilli and cilia, have highlighted the role of Prominin-1 as an organizer of plasma membrane protrusions. Prominin-1 acts on the architecture of microvilli and other actin-driven protrusions such as filopodia by interacting with phosphoinositide 3-kinase and actin-related protein-2/3 complex. These interactions are dependent on the phosphorylation of tyrosine 828 in Prominin-1 and are impacted by mutations in its ganglioside-binding site. Prominin-1 has an influence on assembly-disassembly dynamics of primary cilium by interacting with ADP-ribosylation factor-like 13B ATPase and histone deacetylase 6, respectively. Such Prominin-1 interactions control the fate of stem cells.
Biology of Stem (Cancer Stem) Cells
We are also interested in understanding the remodeling processes of the plasma membrane of stem and cancer stem cells during various biological processes such as differentiation, cell migration and intercellular communication. We are using cells that originate from various sources notably the hematopoietic system. We developed experimental conditions that mimic the stem cell microenvironment. For example, the bone marrow stem cell niche has been reproduced using primary human hematopoietic stem and progenitor cells growing on human multipotent mesenchymal stromal cells as a feeder cell layer. Using these biological tools and Prominin-1 as a biomarker of plasma membrane protrusions, we have highlighted novel and unexpected biological characteristics of primitive cells, which include i) asymmetric cell division of stem and progenitor cells as well as cancer cells; ii) release of Prominin-1+ extracellular membrane vesicles (EVs) (e.g., ectosomes/microvesicles and exosomes) during the stem cell differentiation, and iii) formation of tunneling nanotubes between hematopoietic stem and progenitor cells or their leukemic counterparts as a new mechanism of intercellular communication. In a near future, we would like to identify which types of biological information other than Prominin-1 are shared or exchanged by means of EVs or tunneling nanotubes and determine whether transferred biomaterials influence the biochemistry of the recipient cells. The molecular mechanism underlying the formation of tunneling nanotubes will be the subject of our future research.
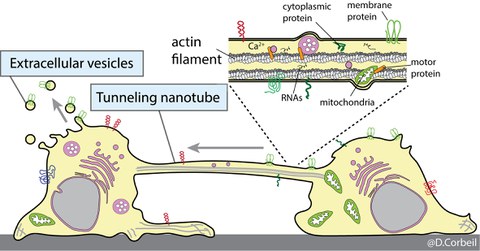
Stem and progenitor cells communicate with each other and their microenvironment either through the release of extracellular vesicles containing the Prominin-1 stem cell marker or through the exchange of this marker by the formation of tunneling nanotubes
