May 25, 2018
If solubility is the problem - Mechanochemistry is the solution
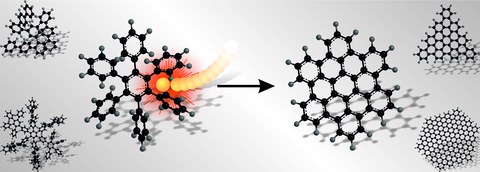
Mechanical energy provided by the collision of milling ball in planetary ball mills allows to synthesize nanographene structures under environmentally friendly and solvent-free reaction conditions.
Chemists from TU Dresden synthesize supersized nanographenes with ball milling
Chemist Dr. Lars Borchardt and his team at TU Dresden recently achieved a huge breakthrough in the synthesis of nanographenes. Because of their unique electrical, thermal and mechanical characteristics, the carbon modification graphene and its little brothers the nanographenes are known as a very promising material for applications in electronics, sensor technology and energy storage. However, since the synthesis of nanographenes and graphene nanoribbons is still rather expensive and environmentally unsustainable, there are only few industrial applications. Dr. Borchardt’s innovative method of a mechanochemical synthesis of nanographenes has certainly paved the way for a safer, simpler and more sustainable route for the synthesis of alternative electronic and solar energy materials.
Ball mills instead of solvents – this is the starting point of the research of Dr. Lars Borchardt and his junior research group „Mechanocarb“ at the Faculty of Chemistry and Food Chemistry at TU Dresden since 2015. The group is funded by the Federal Ministry of Education and Reserach (BMBF) and is a project of the funding initiative
„Materialforschung für die Energiewende“. Their joint aim is to establish mechanochemistry as a resource-, energy- and time-efficient synthesis method towards carbon-based electrode materials. PhD student Sven Grätz recently succeeded once more in proving that they are on the right track: the results of his dissertation on the mechanochemical Scholl reaction were published in the renowned online journal Chemical Communications.
It may seem paradoxal to imagine that the destructive forces of a ball mill can help creating complex molecules. However, Borchardt and his team have done just that. Highly aromatic molecular systems (highly aromatic in chemistry means systems with a high number of conjugated bonds that are very stable) such as nanographenes are known for their poor solubility. Therefore, they are difficult to synthesize in traditional chemical methods, which require a solvent. The Borchardt group exclusively works with the intense mechanical forces of ball mills. The huge forces in the mills initiate a chemical reaction in which a hexaphenylbenzene precursor is converted into a completly aromatic system. Not only does this method represent a much simpler, safer and more sustainable alternative to conventional chemical syntheses, it also opens up new ways: „We can also broaden the feasibility of this famous reaction towards molecules that are insoluble, “ explains Borchardt.
The TUD scientists managed to synthesize the triangular shaped C60 as well as C222 benchmark nanographenes within very short time and with comparably little effort. Now they continue their mechanochemical research with the aim of producing even larger molecules such as graphene nanoribbons which are adaptable for application. The recent findings of the Borchardt group will certainly contribute new aspects to the search for new electronic and solar energy material and also to resolving some of the hindrances of chemical synthesis by eliminating solvents.
Original publication:
S Grätz et al, Chem. Commun., 2018, DOI: 10.1039/c8cc01993b (This article is free to access until 12 June 2018.)
Media inquiries:
Dr. Lars Borchardt
Tel.: +49 (0) 351 463-34960
Homepage: www.borchardt-group.com
