Research
Research in the Bringmann lab: control and functions of sleep
Sleep is a systemic physiological state that is essential for an organism's survival. Sleep affects virtually all physiological processes and plays a prominent role in development, tissue homeostasis, regenerative processes, and longevity [1, 2]. Insomnia and sleep disorders are widespread in modern societies and pose a massive unsolved health problem [3]. All animals with a nervous system sleep, suggesting that this physiological state emerged early in evolution [4]. To understand sleep, we have to understand how it is controlled and how it exerts its functions at the molecular and cellular level. To solve these questions, our lab has embarked on a long-term research journey to first figure out how sleep is controlled in C. elegans. We then use the molecular knowledge about sleep to genetically deprive C. elegans of sleep and thus to understand the molecular mechanisms underlying its cell biological and biochemical functions. We finally use the molecular sleep information from C. elegans as a roadmap to also understand mammalian sleep. In summary, we have three major research aims that we are pursuing:
Aim 1. Understand molecular mechanisms of sleep regulation in C. elegans
Aim 2. Understand the vital functions of sleep at the molecular level in C. elegans
Aim 3. Use C. elegans sleep knowledge as a roadmap to understand mammalian sleep
AIM 1. Understand molecular mechanisms of sleep regulation in C. elegans
Little is known about how sleep is controlled at the molecular level. Understanding the control mechanisms of sleep is important, as it will provide entry points for manipulating sleep to study its functions and to develop treatments for sleep disorders. Molecular information on sleep regulation will facilitate translation of findings into other organisms and for developing therapies for human sleep disorders. Sleep in mammals is induced by GABAergic peptidergic neurons that depolarize at the onset of sleep and are thus called "sleep-active” neurons [5]. However, the molecular control of sleep-active neurons is not well understood. If we want to comprehend how sleep is controlled, we need to understand how the depolarization of sleep-active neurons is regulated. This includes the biochemical pathways that signal sleep need after prolonged wakefulness and an increased demand for sleep during development, regeneration, or illness. In order to understand the molecular biology of sleep control, we set out to develop C. elegans as a model to study sleep-active neurons. Because sleep is vital, it is found in every animal that has a nervous system ranging from jellyfish to humans [6]. Sleep can be identified using behavioral criteria such as reduced responsiveness to stimulation, reversibility, and homeostatic regulation [7]. C. elegans shows sleeping behavior across life stages and during various conditions. Our lab has been the first to do systematic genetic screening for sleep genes in C. elegans [8]. We found a set of genes required for sleep and the analysis of these genes led to the identification of a single sleep-active neuron in C. elegans called RIS. The RIS neuron behaves like mammalian sleep-active neurons: it depolarizes at the onset of sleep, and actively induces sleep by inhibiting wake circuits through the release of GABA and inhibitory neuropeptides (Figure 1-2) [9].
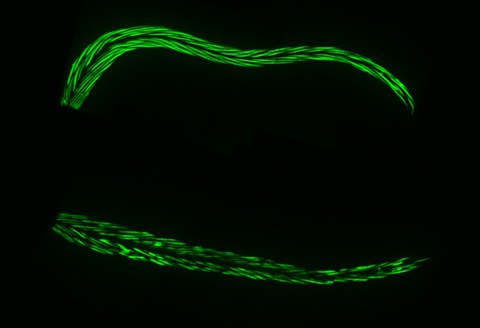
Figure 1. The RIS neuron is active during sleep. Here, the activity of all neurons was visualized with a genetically-encoded calcium indicator called GCaMP (green fluorescence, the brighter the green light, the more active the neuron). Top: during wakefulness, most of the neurons are active for most of the time. Bottom: during sleep, most neurons are inactive except the RIS neuron, which activates specifically during sleep.
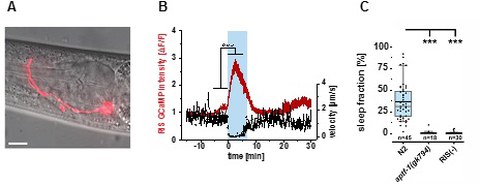
Figure 2. The sleep-active RIS neuron induces sleep. A) The single RIS sleep-active neuron (red). B) RIS depolarizes during sleep bouts. C) RIS is required for sleep bouts.
The identification of a single sleep-active neuron in C. elegans provides a unique opportunity to study the molecular biology of sleep control in an accessible model system. Current work in our lab focuses on signaling pathways that activate RIS including antimicrobial peptides produced in response to wounding or infection [10], EGF signaling that is activated during development and stress [11], as well as conserved food-sensing longevity-promoting pathways such as FOXO/DAF-16 and AMP-activated kinase [12].
AIM 2. Understand the vital functions of sleep at the molecular level in C. elegans
The knowledge of how sleep is controlled allows the manipulation of this state and thus the study of the consequences of lost or increased sleep. Because sleep in C. elegans requires crucially the single RIS neuron, sleep can be easily and efficiently removed from this system by ablating RIS. Conversely, increasing the activity of this neuron, for instance using optogenetics, can be used to increase sleep. Thus, sleep can be controlled through manipulation of sleep-active neurons, which allows testing for the phenotypes caused by either loss of sleep or increased sleep.
Our sleepless C. elegans system provides a unique chance to study the consequences of sleep loss and thus molecular sleep functions in an accessible model animal. Our lab started analyzing sleepless worms and we found that sleep becomes essential for slowing down the progression of aging phenotypes [12], and for surviving injury [10]. A highly conserved network of genes controls aging [7]. We showed that this aging gene network controls the activity of the sleep-active RIS neuron through FoxO and AMP-activated kinase to induce sleep. The depolarization of RIS in turn induces sleep to slow the progression of aging phenotypes. Thus, rather than only conserving energy for later use, sleep is part of a protective program induced by anti-aging genes to counteract cellular demise (Figure 3-4) [12].
Figure 3. RIS counteracts the progression of aging phenotypes. Cartoon summary of the link between aging, starvation, and sleep. Nutrient starvation triggers arrest through a conserved longevity gene network that depolarizes RIS. RIS in turn counteracts aging phenotype progression, and thus prevents death. How RIS acts to slow aging phenotype progression is subject to current studies.
Figure 4. Impairing the sleep-active RIS neuron impairs health and increases aging phenotypes. The worm on top expresses a muscle myosin reporter and sleeps regularly. It is healthy and its muscle fibers are well preserved. The worm on the bottom of the picture lacks sleep because it carries a mutation that impairs the sleep-active RIS neuron. As a consequence of RIS impairment, muscle fibers deteriorate more quickly. Such muscle deterioration is typically seen during aging across species, including in humans, where this condition is called sarcopenia.
AIM 3. Use C. elegans sleep knowledge as a roadmap to understand mammalian sleep
Sleep is widespread among animals and has been found in all animals that have a nervous system. Molecular genetics and circuit analysis have shown that mechanisms underlying sleep regulation are highly conserved across species. For example, sleep is generally induced by sleep-active sleep-promoting neurons that directly inhibit wakefulness circuits through GABA and neuropeptides [4]. Also, genes controlling sleep are conserved, an example being AP2 transcription factors [8, 13-15]. Our lab studies mouse sleep in mutants of genes that we have identified in worms. We have started this line of research by studying the role of AP2 transcription factors in mouse sleep (Figure 5). In mammals, AP2 diversified into several TFAP2 proteins that are encoded by separated genes (TFAB2A, TFAB2b, etc.) Our results of EEG measurements in AP2 mutant mice showed that AP-2 transcription factors control sleep behavior also in mice, but that the role of the AP-2 genes functionally diversified to allow for a bidirectional control of sleep quality with TFAP2B promoting sleep and TFAP2A inhibiting sleep. This divergence of AP-2 transcription factor functions might perhaps have supported the evolution of more complex types of sleep [16].
Figure 5: A C57BL/6 mouse sleeping in our lab.
We are studying how genes that we identified to control sleep in C. elegans control mammalian sleep.
References
1. Cirelli, C., and Tononi, G. (2008). Is sleep essential? PLoS biology 6, e216.
2. Mignot, E. (2008). Why we sleep: the temporal organization of recovery. PLoS biology 6, e106.
4. Bringmann, H. (2018). Sleep-Active Neurons: Conserved Motors of Sleep. Genetics 208, 1279-1289.