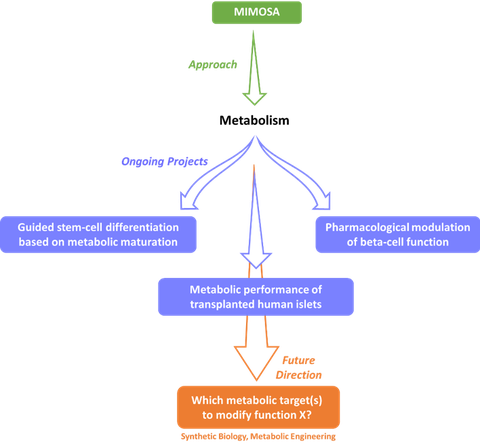
Research Focus
The interest in metabolism has been revived in recent years due in part to our evolving understanding of its role in the regulation of cellular homeostasis. It is now evident that metabolism acts also as a transducer of external stimuli, regulating signaling cascades, post-translational modification of protein activity and even gene expression. This metabolically-driven maintenance of cellular homeostasis is not consistent with the often assumed rigid and linear view of chemical reactions. Rather, it is a highly flexible, interconnected and responsive network that relies on extensive and highly nuanced mechanisms of regulation to direct the flow of metabolites throughout the cell.
Therefore, the general aim of our group is to understand how this metabolic regulation is affected, thus enabling our ability to modulate cellular function with targeted metabolic manipulations.
Approach
We use stable isotopes to maximize the capture of the dynamic nature of metabolism. The transfer of mass isotopes, such as 13C, across metabolites is highly specific, well characterized and provides a critical glimpse into the magnitude and direction of individual reactions. This work is based on the previously developed MIMOSA (Mass-Isotopomer Multi-Ordinate Spectral Analysis). MIMOSA takes advantage of the high sensitivity of mass spectrometry (MS) and extracts position-specific labeling patterns from the otherwise unspecific MS data (Alves et al, Cell Metab, 2015). Initially developed to study central carbon metabolism, this platform is in continuous development as we can expand it to include other pathways or highlight specific reactions depending on the needs of each project. This approach is also used in combination with techniques of computational analysis and molecular biology to extract the most detail possible from each project.
Ongoing Projects
Our active projects are centered on the development of therapeutic options for Diabetes. Diabetes is a chronic disease that affects millions of people worldwide. Regardless of the type of Diabetes, this disease is associated with profound metabolic alterations. Currently, our focus is on understanding the role of metabolism in the regulation of pancreatic beta-cell physiology. Pancreatic beta-cells are responsible for the secretion of insulin in response to glucose stimulation and represent a major factor in managing and delaying the offset of both Type 1 and Type 2 Diabetes. The scientific excellence of the campus is crucial in fomenting collaborations with other groups and expanding the clinical scope of our research. Therefore, our projects involve studying:
- the link between glucose metabolism and insulin secretion. We use a combination of metabolic flux screening and computational analysis to identify the optimal targets for pharmacological modulation of insulin secretion;
- the role of metabolic performance in the outcome of human islet transplantation.
- the interaction between metabolism and epigenetics during the process of stem-cell differentiation into beta-cells.
Future Direction
In addition to the clinical relevance of the above-mentioned projects, beta-cells provide a great model to study metabolism. The rapid response of insulin secretion following changes in glucose concentration are not consistent with changes in transcriptional and translational events but rather represent the unique properties of each individual enzyme. This information is crucial to understand how a metabolic system is regulated. Our ultimate goal is to combine this knowledge with other disciplines (systems biology, epigenetics, proteomics, etc.) to be in a position to identify which metabolic targets need to be modulated in order to affect a specific function in any cell. We welcome applicants that share this vision and wish to apply it to beta-cells or other models.