Research Focus
Cell fate decisions of pancreas progenitor and stem cells
We are interested in understanding how extracellular signals and intrinsic genetic programs interact to dictate cell fate decisions in stem and progenitor cells and establish mature phenotypes. Our main focus is the development of the endocrine lineage in the pancreas and the conversion of human pluripotent stem (hPS) cells into functional beta cells. Key questions that we are addressing concern the signals that guide cell transitions during pancreas differentiation and the regulators of the timing of these transitions.
We have identified a new signal, sphingosine-1-phosphate, which plays a conserved role in the aggregation of endocrine cells to form islets. The same signaling pathway mediates survival of acinar and endocrine progenitors and triggers their differentiation through stabilization of YAP and attenuation of Notch signaling. Extending these findings, we have found that effectors of GPCR signaling regulate lineage allocation during pancreas development.
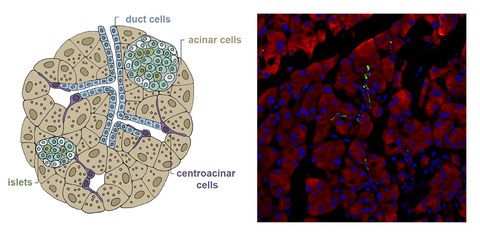
Additionally, we have found that Aldh1b1, encoding a mitochondrial enzyme, regulates the timing of differentiation in the developing pancreas. The gene is expressed in all pancreatic progenitors during development and in the rare centroacinar cells of the adult pancreas, but not in differentiated pancreatic cells. Aldh1b1 elimination during development accelerated differentiation and compromised functionality of the adult beta cells. Recent findings suggest that Aldh1b1 is a metabolic regulator that helps determine chromatin accessibility of the progenitors. Genetic lineage tracing showed that the rare Aldh1b1 expressing cells in the adult pancreas give rise to cells of all three pancreatic lineages and that Aldh1b1 is required for the development of pancreatic cancer.
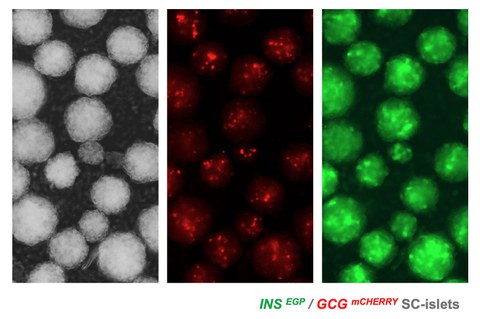
We are taking advantage of these findings to expand hPS cell derived pancreatic progenitors and efficiently convert them into pancreatic islets containing mature beta cells (SC-islets). To accelerate the process of identifying optimal conditions we have developed a series of hPS cell reporter lines and an automated imaging pipeline. Additionally, we now include endothelial cells and pericytes in the differentiation of SC-islets to accelerate maturation and enhance their integration after transplantation. Other ongoing work suggested that Aldh1b1-expressing centroacinar cells might be tumor initiating cells and we are exploring these findings to understand the early stages in the development of the disease.
Future Projects and Goals
- Identify signaling requirements and the underlying molecular mechanisms for the different pancreatic lineages
- Elucidate the role of metabolism in the differentiation of pancreatic progenitors and beta cell functionality
- Explore the role of adult pancreas progenitor cells in cancer development
- Use directed differentiation of hPS cells into pancreatic islets to understand human endocrine development and develop cell therapies for diabetes