Research Focus
Our lab focuses on understanding how species like the Mexican salamander, can regenerate complex tissues such as the limb, but also organs such as the pancreas. For example, it has been shown that limb regeneration requires the participation of stem or progenitor cells, but also the reprogramming of mature cells. Yet, some cells do not participate in regeneration, highlighting the diversity in cellular mechanisms that an organism employs to restore lost tissue. Our main goal is to understand how individual tissues respond to an injury to further understand their interaction. The fine-tuned coordination of the individual tissue regeneration and the interaction with other tissue types could be the key for successful regeneration and formation of new organs or structures.
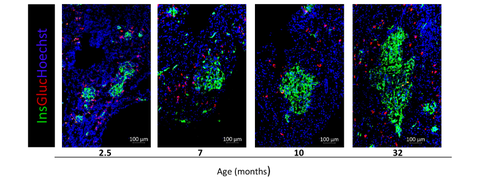
Figure 1. Immunohistochemistry of axolotl pancreas during larval stages (2.5, 7 months) and adult stages (10 and 32 months).
Another aspect that we study, is how the organism responds to the high energy demand that is required to regenerate a whole limb. We have found that metabolic organs respond by adjusting the amount of stored lipids, but also those in circulation. We want to understand how the local environment of the injured tissue conveys systemic signals that alter metabolism to ensure regeneration.
Approach
Axolotl verfügen über eine enorme Regenerationsfähigkeit während ihrer gesamten Lebensspanne, was sie für die Wissenschaft sehr interessant macht. Da die Haut außerdem nicht pigmentiert ist, lassen sich die Zellen der Tiere in Echtzeit sichtbar machen. Wir untersuchen die dabei zugrunde liegenden Regenerationsmechanismen, indem wir genomische Werkzeuge, hochauflösende In-vivo-Bildgebung, Immunohistochemie und Verfahren einsetzen, die zum Beispiel auch den Nachweis spezifischer Moleküle im Blutkreislauf ermöglichen.
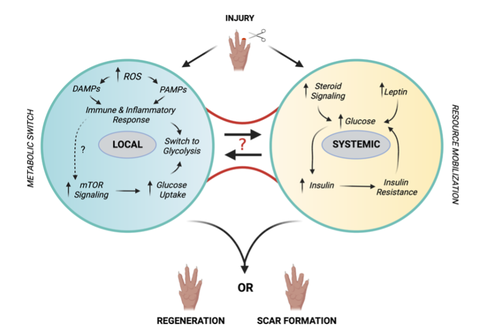
Figure 2. Schematic representation of possible signaling networks triggered by injury and affecting distant organs.
Ongoing Projects
We study pancreas regeneration in the axolotl. We aim to study how a cryoinjury triggers cellular mechanism to replace endocrine cells. In addition, we are stablishing genetic tools to ablate beta-cells, as this will allow cross-species comparisons.
We study how the organism sustains the energetic demands of the regenerative process; what signals are produced at the injury site and how the metabolic organs respond to maintain homeostasis while a fast growth is sustained at the regenerating site.
We aim to understand skeletal regeneration, and how remodeling of existing tissues primes the integration of the newly regenerated structures. Functional integration of the old and new tissue is crucial and yet, we know little of the molecular mechanisms governing this process.
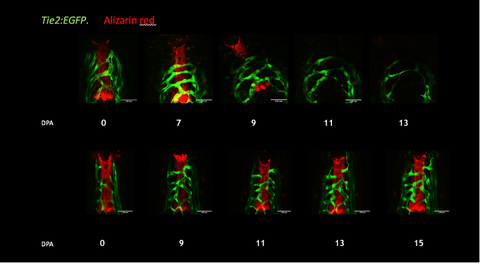
Figure 3. Blood vessel remodeling during digit regeneration in the axolotl (top panel) compared to intact digits (lower panel). Alizarin red staining labels the mineralized skeleton.
Future Directions
Our long-term goal is to build an understanding of regeneration, how the molecular and cellular building blocks come together to coordinate the formation of new tissue. This will facilitate bridging our discoveries from the axolotl into viable mammalian therapeutics that can impact human regeneration.
