Oct 23, 2017
Introduction novel system for in vitro analyses of zebrafish oligodendrocyte progenitor cells

Dr. Michell M. Reimer
Dr. Michell M. Reimer, group leader at the Center for Regenerative Therapies Dresden (CRTD), Cluster of Excellence at the TU Dresden, and his team introduce a novel, easy-to-use, and highly reproducible OPC culture platform for adult zebrafish cells. This system will help to unravel the molecular and cellular programs that enable zebrafish to functionally regenerate spinal cord injuries. The results of this study have been published in the scientific journal Frontiers in Cellular Neuroscience.
Spinal cord injuries result from a blunt or penetrating trauma. This is generally caused by accidents that occur during sport activities or when driving. Injuries of the spinal cord can lead to extreme pain (e.g. pressure in the head, neck or back), the loss of sensation (e.g. in fingers or feet), the loss of control over different parts of the body, an abnormal sense of balance and many other symptoms. According to the World Health Organization (WHO)*, as many as 500,000 people suffer from spinal cord injuries each year*. Humans do not regain spinal cord function after injury. However, zebrafish have the remarkable ability to functionally recover from spinal cord injury. They repair injured connections, replace damaged motor neurons and oligodendrocytes, enabling them to regain full movement within six weeks after injury.
The study introduced here focused on a population of support cells in the spinal cord that helps to protect surviving nerve cells (neurons) after injury: oligodendrocytes and their precursor cells. Oligodendrocytes, the cells that are known to produce the myelin sheaths which enable saltatory conduction of action potentials along the myelinated axons, are modulators of signal transmission along neuronal connections (axons) and also promote neuronal survival by providing metabolic support. Oligodendrocyte death, occurring after a spinal cord injury, activates a process called de-myelination that results first in damage to surviving neuronal connections and finally in death of the affected neurons. Although lost mature oligodendrocytes can principally be replaced by resident oligodendrocyte progenitor cells (OPCs) this does not happen sufficiently enough in the human spinal cord after injury. Improving recruitment, activation and differentiation of OPCs is therefore hypothesised to improve functional outcome after a spinal cord injury in humans.
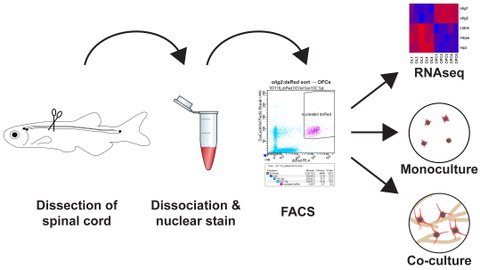
Here Dr. Reimer and his team asked the question, ‘what happens to mature oligodendrocytes after a spinal cord injury in adult zebrafish?’. They found that, like in humans, oligodendrocytes near a spinal cord injury site are massively lost within a week. However, two weeks after injury they found that the oligodendrocyte population was largely re-established, showing the remarkable regenerative capacity of the adult zebrafish spinal cord. These results placed the resident OPC population in the focus of interest: what are the signals that control and enable the activation of these precursor cells in the adult zebrafish spinal cord? Dr. Reimer and his team decided to establish a novel in vitro platform to analyse zebrafish OPCs independently of the body, as this enables better control over the cells and opens up the possibility for novel methods of analysis. They developed a streamlined and fast, though inexpensive, method that allows direct access to a pure and vital population of zebrafish OPCs in less than 2 hours. This simple protocol is based on automated fluorescent activated cell sorting (FACS) of OPCs. Using novel culture conditions Dr. Reimer’s team has shown it is now possible to maintain the cells for 16 days in vitro. Finally, they demonstrated that zebrafish OPCs differentiate into mature oligodendrocytes when cultured together with human motor neurons, differentiated from induced pluripotent stem cells. This shows that the basic mechanisms of oligodendrocyte differentiation are conserved across species and that understanding the regulation of zebrafish OPCs can contribute to the development of new treatment for human diseases.
As a next step, Dr. Reimer’s research team intend to analyse the effect of different drugs on zebrafish OPCs in order to potentially identify a method to improve functional spinal cord repair in humans.
Before becoming a research group leader at the CRTD in 2014 (for Regulation of developmental and regenerative processes in the spinal cord), the biologist Michell Reimer worked as a Post-Doctoral Fellow at the Centre for Neuroregeneration and the Centre for Cognitive and Neural Systems at the University of Edinburgh (UK) since 2009. From 2005-2008, Michell Reimer completed his PhD in the field of neuroscience at the Centre for Neuroscience Research, University of Edinburgh. http://www.who.int/mediacentre/news/releases/2013/spinal-cord-injury-20131202/en/
Publication
Title: Primary Spinal OPC Culture System from Adult Zebrafish to Study Oligodendrocyte Differentiation In Vitro
Kroehne V., Tsata V., Marrone L., Fröb C., Reinhardt S., Gompf A., Dahl A., Sterneckert J. Reimer M.M.
DOI: 10.3389/fncel.2017.00284
Media inquiries:
Franziska Clauß, M.A.
Press Officer
Tel.: +49 (0) 351 458 82065
Founded in 2006, the DFG Research Center for Regenerative Therapies Dresden (CRTD), Cluster of Excellence at the TU Dresden aims to explore the human body's regenerative potential and to develop regenerative therapies for hitherto incurable diseases. Key disease areas of research include haematology and immunology, diabetes, neurodegenerative diseases, and bone regeneration. The CRTD is part of the Center for Molecular and Cellular Bioengineering (CMCB), a central institution of the Technische Universität Dresden. http://www.crt-dresden.de / http://www.tu-dresden.de/cmcb