Karyotyping by G-banding
Human or primate iPSCs are expanded to 10 cm culture dishes per sample. Required are subconfluent cultures in exponential growth phase, ca. 1 day before regular passaging density. The cells are arrested in metaphase by colcemid block for 2,5 h, harvested and fixed. After Giemsa staining the metaphase spreads are analyzed at the Institute of Clinical Genetics, University Hospital Carl Gustav Carus Dresden, or at the certified Molecular Cytogenetics laboratory of Jena University Hospital.
The mean resolution of G-banding is 200 bands per haploid chromosome set (comparable to human bone marrow quality). Submicroscopic changes (microdeletions/microduplications) and changes smaller than 10 Mb cannot be excluded by this method. Mosaicism is only detected and mentioned in the karyotype formula if chromosomal losses appear >3 times or structural aberrations and gains are observed >2 times. Composite karyotype [cp] is given from 20 metaphase spreads each.
The karyotype of regularly passaged iPSC lines should be checked every 20 passages. In addition, one should check the karyotype of newly derived lines after reprogramming, genome engineering, if bottlenecks in the culture occurred by increased selection, changing culture conditions and after subcloning.
The facility recommends to establish an early master cell bank and working cell banks from every iPSC line by cryopreservation of reasonable amounts of vials of the same passage parallel to karyotyping. Experiments should always be started from the same working cell bank within 20 passages. After maximum 20 passages the cells in culture should be replaced by thawing a fresh vial from the working cell bank.
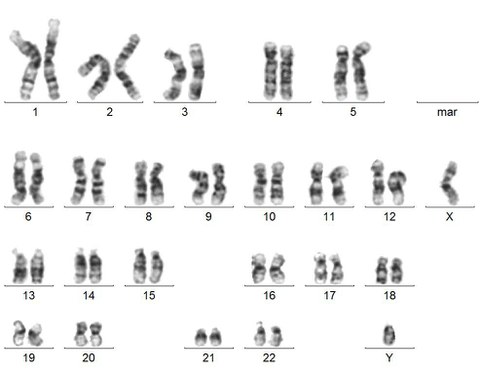
Karyotyping by G-banding. An example metaphase spread of Giemsa stained chromosomes is depicted for the CRTD1 hiPSC line with an intact chromosome set 46,XY[cp20].
